US Pharm. 2013;38(1):HS-12-HS-16.
ABSTRACT: Generalized convulsive status epilepticus (GCSE) is a neurologic emergency requiring immediate medical attention. Pharmacists are front-line health care providers when it comes to the management of GCSE, and they must be equipped with an in-depth knowledge of the appropriate agents used in its management, including benzodiazepines (BZDs) and antiepileptic drugs (AEDs). A BZD should be used initially to control seizure activity, with an AED given as soon as possible after BZD administration. During any GCSE presentation, the route, adverse-effect profile, monitoring necessity, and proven efficacy of each agent under consideration should be evaluated to determine the appropriate BZD and AED.
Generalized convulsive status epilepticus (GCSE), a neurologic emergency requiring immediate medical attention, has a reported fatality rate of up to 30% to 40% after diagnosis.1-3 GCSE occurs in children, as well as in adults; however, this article will focus only on management in adults. Other forms of status epilepticus (SE) exist, such as nonconvulsive status epilepticus (NCSE), but they are less common than GCSE and will not be discussed here.4
The definition of SE is constantly fluctuating, which creates a dilemma in the determination of whether a patient fits the clinical picture of SE. Historically, the disorder has been defined as seizure activity for more than 30 minutes leading to irreversible neuronal damage, but the duration of seizure activity for diagnosis has been continually shortened.1,2,5-9 Most recently, Brophy et al developed guidelines that define SE as either 5 or more minutes of continuous seizure activity or recurrent seizure activity without an appropriate return to baseline.10 Physiological alterations—including internalization of gamma-aminobutyric acid (GABA)A receptors and increased sensitivity to excitatory neurotransmitters—occur that make it unlikely for seizure activity lasting longer than 5 minutes to stop spontaneously, which supports the aforementioned shortened timeframe.11-14
Because of the emergent nature of GCSE, prospective, randomized data regarding appropriate pharmacologic management are limited. Guidelines have been developed by the Neurocritical Care Society and the European Federation of Neurological Societies for appropriate pharmacotherapeutic management of GCSE.1,10 Pharmacists are front-line health care providers in the management of GCSE, and knowledge of these guidelines and proper medication use in specific GCSE patient populations should not be underestimated or undervalued. This requires an in-depth evaluation of each available drug in terms of dosing, route, common or serious adverse effects (AEs), and data supporting efficacy for GCSE. The following review focuses on these aspects so that hospital-based pharmacists will be fully armed with the knowledge to make appropriate initial pharmacotherapy decisions when managing GCSE in adult patients.
PHARMACOTHERAPEUTIC MANAGEMENT
Initially, if the cause of GCSE is known (e.g., hypoglycemia requiring glucose and thiamine to reverse the hypoglycemia while preventing Wernicke’s encephalopathy), reversal of the cause is of primary importance.2,6 Other commonly associated reversible causes of GCSE include acidosis, hypoxia, and electrolyte disturbances (including hyponatremia and hyperkalemia).2,14 Additionally, subtherapeutic antiepileptic drug (AED) levels in epileptic patients presenting with GCSE should be assessed and immediately addressed.2,7,10
In concert with the evaluation of reversible causes, aggressive treatment should be initiated as soon as possible, without a delay for diagnostic workup.13 The current pharmacotherapy recommendations for GCSE stress the use of benzodiazepines (BZDs) and AEDs through nonenteral routes of administration (e.g., IV, intramuscular [IM], per rectum [PR]); recommended dosing is summarized in TABLE 1. Control of SE should be gained within 60 minutes of the initial insult.10 Unfortunately, current literature suggests that only 30% to 60% of GCSE patients respond to the initial agent or the second agent selected.1,3,10,15
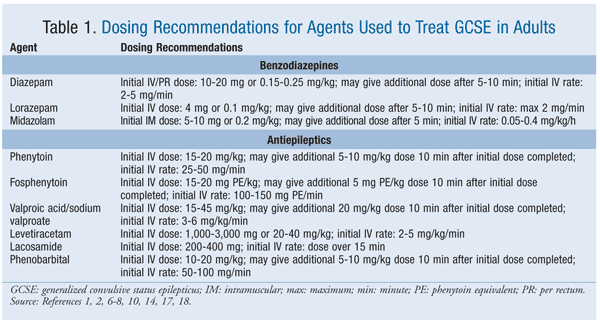
The roles of individual agents in GCSE management remain ill-defined despite the aforementioned guidelines.1,3,10 The VA Cooperative Study (Treiman et al) attempted to determine the most effective regimen for GCSE control.15 However, this study examined four treatment arms containing diazepam plus phenytoin, lorazepam, phenytoin, or phenobarbital, neglecting newer AEDs as well as the commonly used combination of lorazepam plus an AED.15 Additionally, patients with SE often require three or more AEDs for managing GCSE, presenting another challenge for appropriate medication selection.3 The following sections will focus on agents available in nonenteral routes for the management of SE.
Benzodiazepines
Three BZDs are primarily used in the management of GCSE: diazepam, lorazepam, and midazolam. All work at the postsynaptic GABA neuron in the central nervous system (CNS).16 These agents inhibit neuronal excitability through increased permeability to chloride ions, leading to hyperpolarization and stabilization at the neuron. Success rates of BZDs for SE cessation range from 60% to 90%, and the vast majority of patients in recent studies received a BZD as initial therapeutic treatment.3,6,17 Additionally, the aforementioned VA Cooperative Study showed the inferiority of phenytoin to lorazepam, thus strengthening the recommendation that BZDs are the appropriate initial medication for any GCSE cessation attempt.15
Diazepam: Of the three BZDs, diazepam is the most lipid soluble and enters the CNS quickly. However, it is rapidly redistributed (usually ≤30 minutes) outside the CNS, which somewhat limits its utility. Diazepam’s elimination half-life is roughly 24 to 48 hours, creating the possibility of accumulation if repeated administration is needed; noted AEs include respiratory depression, hypotension, and sedation.2,6,13,16 One benefit of diazepam is its availability in both IV and PR routes. Multiple studies have shown the superiority of diazepam over placebo for SE, but superiority over other pharmacologic agents is not well documented.6
Lorazepam: This agent is considered by many practitioners to be the BZD of choice in the management of GCSE.2,6 While it is less lipid soluble than diazepam, it redistributes from the CNS more slowly than diazepam, thus conferring a longer duration of action and a reduced rate of recurrent seizures. While the AEs seen with lorazepam are similar to those for diazepam, the reduced lipid solubility lessens the risks of hypotension and respiratory depression. Additionally, lorazepam binds to GABA receptors with greater affinity, providing a relatively extended anticonvulsant effect of 6 to 12 hours.2,6,16 However, tolerance to lorazepam develops rapidly, so subsequent doses may be less effective than initial doses. A recent retrospective analysis found that patients who initially received lorazepam alone or with an AED had a significantly shorter time to SE resolution, backing the current practice model, which uses lorazepam as the initial agent in GCSE management.3
Midazolam: Commonly used in Europe, midazolam is often the third BZD option considered for initial management of GCSE in the United States, owing to its extremely short half-life (90-150 minutes) and the need for frequent boluses.13,16 Midazolam is available in IV, IM, buccal, intranasal, and PR routes.2,16,18 In children, use of buccal midazolam has been effective and has had no clinically significant AEs, which is an advantage when no IV access is available.6,18 Bolus injections of midazolam tend to be relatively ineffective because of the drug’s short half-life. In the U.S., this agent has primarily been used in refractory SE as an IV infusion, which is beyond the scope of this article.2,13,16
In a comparison of BZDs for the management of SE, Alldredge et al found that seizure cessation occurred in 59.1% of patients given lorazepam 2 to 4 mg and in 42.6% of those given diazepam 5 to 10 mg, with both agents significantly more effective than placebo.19 Additionally, Leppik et al saw no difference in AEs in a comparison of the two agents; seizure-cessation rates with one or two doses of lorazepam or diazepam were 89% and 76%, respectively (not statistically significant).20 Recently, an evaluation of IM midazolam versus IV lorazepam found similar AE profiles and recurrent seizure rates.21 However, the IM midazolam group had a statistically significant improvement (73.4% vs. 63.4%) in seizure-cessation rates upon emergency department arrival and in the proportion of subjects admitted to the ICU.21 These data provide some evidence that IM midazolam is at least as safe and effective as IV lorazepam in the management of prehospital SE.
Phenytoin and Fosphenytoin
Phenytoin works through stabilization of neuronal membranes by increasing the efflux or decreasing the influx of sodium ions, which can prolong the refractory period between neuronal impulses.16 It is often the first AED chosen for GCSE management because of practitioner familiarity with it. Disadvantages of phenytoin include a maximum infusion rate of 50 mg per minute, which can cause difficulty in large patients owing to its weight-based dosing (TABLE 1).16 Because of the increased incidence of hypotension in the elderly population, it is recommended that phenytoin be infused no faster than 25 mg per minute. Additionally, hypotension occurs with the IV formulation regardless of age, possibly because of the presence of propylene glycol (PG), and infusion of either phenytoin or fosphenytoin renders monitoring for cardiac abnormalities (e.g. arrhythmias, hypotension) necessary.10 Other AEs include sedation, respiratory depression, rash, phlebitis, purple glove syndrome, dizziness, and extravasation.6,16,22
Fosphenytoin is a water-soluble prodrug of phenytoin. It contains no PG, theoretically lowering the incidence of hypotension compared with phenytoin. Additionally, fosphenytoin carries a lower risk of purple glove syndrome.6 However, the difference in formulation has not reduced overall AE rates.7,23 One argued benefit is that therapeutic concentrations of phenytoin are achieved more quickly with fosphenytoin, partly because of the faster infusion rate (150 mg phenytoin equivalents [PE]/min). Fosphenytoin also may be administered IM, if IV access is unavailable. One complication of this agent is that dosing is via PEs, with 1.5 mg of fosphenytoin equivalent to 1 mg of phenytoin, possibly creating confusing dosing scenarios.2,16
Valproic Acid/Sodium Valproate
Valproate is believed to work either through increased availability of GABA or through GABA enhancement or mimicry.16 In multiple small studies, valproate was as effective and safe as phenytoin or fosphenytoin for GCSE, primarily after BZDs were given; one study showed a statistically significant improvement in seizure-cessation rate with valproate.4,8,18,22,24-26 In certain European countries, valproic acid is considered an appropriate initial AED selection after BZDs have been given, with the timing of administration being important since efficacy is time dependent.14,22,25 Retrospective data suggest that valproate used after BZDs results in significantly less treatment failure compared with levetiracetam.8 However, valproate patients had significantly fewer acute and deadly etiologies, creating a difficult-to-interpret scenario. Common AEs noted with valproate are hypotension, dizziness, thrombocytopenia, and hepatic dysfunction, but these occur in fewer than 10% of patients and are not rate dependent.18,22,24 Respiratory depression is not seen with valproate, conferring a possible benefit in patients in whom respiratory monitoring is not possible.2-3,18 Of note, valproate is also effective for the management of NCSE.2,4
Levetiracetam
Levetiracetam’s mechanism is unknown but is postulated to be due to activity at synaptic vesicle protein 2A, which may prevent synchronization of epileptiform burst firing and seizure activity.16,22 In recent studies, 10% to 31% of patients received levetiracetam as the second agent chosen in the management of SE and more than 65% were discharged on levetiracetam, evidencing its increased popularity among AED medications in the management of SE.3,8 Multiple small studies have shown excellent efficacy and low toxicity with the use of levetiracetam in GCSE.8,9,18,22,27,28 Additionally, a small study by Misra et al argued that levetiracetam may be an acceptable first-line agent compared with lorazepam because of similar efficacy and a reduced rate of hypotension and respiratory compromise.29 Completed rigorous and comparative clinical trials of levetiracetam’s efficacy and safety are lacking, with most data being observational in nature and involving heterogeneous patient populations.3,14,27
Somnolence, rash, thrombocytopenia, and paradoxical agitation have been noted with levetiracetam, but respiratory depression and hypotension are not present.3,9,27,29 An additional advantage of levetiracetam is the presence of very limited drug-drug interactions owing to its lack of CYP450 metabolism.14 As mentioned earlier, a retrospective observational study found that valproate used after BZDs had significantly less treatment failure compared with levetiracetam.8 Add to that the initial concern that levetiracetam would not be an effective option in SE because of multiple modeling exercises showing a possible delay in CNS entry versus other AEDs used in GCSE, and there is still reason to question the role of levetiracetam in the treatment of GCSE.4,8
Lacosamide
Lacosamide, a newer AED that may work by enhancing inactivation of voltage-dependent sodium channels, thereby reducing excitable neuronal firing, has shown promise in the management of GCSE.16-18,30 Two recent studies had success rates of 44% to 100% in patients receiving lacosamide for termination of SE, with the low end of that range comparable to rates for phenytoin and fosphenytoin.6,17,30 Additionally, a subgroup analysis showed that the earlier lacosamide was given, the greater the success rate of SE cessation.17 As with levetiracetam, there is a lack of rigorous, comparative clinical trials of lacosamide, and further evaluation is necessary before its role in the management of GCSE can be determined.
Rash and pruritus have been noted as possible AEs with lacosamide.30 However, cardiorespiratory depression and hypotension were not reported, possibly representing an added benefit compared with more commonly used agents.3,17
Phenobarbital
Phenobarbital is similar to BZDs in that its mechanism is exerted at GABAA receptors. However, it binds to a different location on the receptor.7,16 Phenobarbital has shown 60% to 70% efficacy in SE cessation, but because of its significant AE profile it is typically used second-line after failure of BZDs and more commonly used AEDs.6,31 AEs seen with phenobarbital include rash, sedation, and respiratory depression that may require airway protection. Hypotension also is common owing to its dilution in PG. This dilution also raises concern about serious AEs, such as renal failure and myocardial depression.2,6,16
CONCLUSION
In general, lorazepam is the preferred IV BZD and should be given as soon as GCSE has been diagnosed. Diazepam and midazolam should be used in cases in which lorazepam is not a viable option owing to medication unavailability or the need for non-IV nonenteral administration. An IV AED should be infused immediately after lorazepam administration. There is no definitive second agent in GCSE; correct AED selection incorporates many factors, such as monitoring and product availability. While an older AED like phenytoin is commonly selected based on practitioner familiarity, newer agents are frequently used in current GCSE treatment algorithms. Randomized, controlled trials are necessary to determine the most appropriate initial management of GCSE, as well as which AED should be initially selected, but they are difficult to complete because of the emergent nature of GCSE.8,18 Until trials of this type are completed, pharmacists will play a vital role in AED education, including AEs, proven efficacy, and dosing recommendations for each agent under consideration.
REFERENCES
1. Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17:348-355.
2. Nair PP, Kalita J, Misra UK. Status epilepticus: why, what, and how. J Postgrad Med. 2011;57:242-252.
3. Cook AM, Castle A, Green A, et al. Practice variations in the management of status epilepticus. Neurocrit Care. 2012;17:24-30.
4. Wheless JW, Treiman DM. The role of the newer antiepileptic drugs
in the treatment of generalized convulsive status epilepticus. Epilepsia. 2008;49(suppl 9):74-78.
5. Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120-122.
6. Walker M. Status epilepticus: an evidence based guide. BMJ. 2005;331:673-677.
7. Nandhagopal R. Generalised convulsive status epilepticus: an overview. Postgrad Med J. 2006;82:723-732.
8. Alvarez V, Januel JM, Burnand B, Rossetti AO. Second-line status
epilepticus treatment: comparison of phenytoin, valproate, and
levetiracetam. Epilepsia. 2011;52:1292-1296.
9. Aiguabella M, Falip M, Villanueva V, et al. Efficacy of
intravenous levetiracetam as an add-on treatment in status epilepticus: a
multicentric observational study. Seizure. 2011;20:60-64.
10. Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3-23.
11. Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724-7733.
12. Goodkin HP, Joshi S, Kozhemyakin M, Kapur J. Impact of receptor changes on treatment of status epilepticus. Epilepsia. 2007;48(suppl 8):14-15.
13. Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246-256.
14. Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of
status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(suppl 9):63-73.
15. Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. N Engl J Med. 1998;339:792-798.
16. Lexi-Drugs (Lexi-Comp Online). Hudson, OH: Lexi-Comp, Inc; 2012.
17. Kellinghaus C, Berning S, Immisch I, et al. Intravenous lacosamide for treatment of status epilepticus. Acta Neurol Scand. 2011;123:137-141.
18. Shorvon S. The treatment of status epilepticus. Curr Opin Neurol. 2011;24:165-170.
19. Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of
lorazepam, diazepam, and placebo for the treatment of out-of-hospital
status epilepticus. N Engl J Med. 2001;345:631-637.
20. Leppik IE, Derivan AT, Homan RW, et al. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA. 1983;249:1452-1454.
21. Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular
versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591-600.
22. Trinka E. The use of valproate and new antiepileptic drugs in status epilepticus. Epilepsia. 2007;48(suppl 8):49-51.
23. Coplin WM, Rhoney DH, Rebuck JA, et al. Randomized evaluation of
adverse events and length-of-stay with routine emergency department use
of phenytoin or fosphenytoin. Neurol Res. 2002;24:842-848.
24. Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: a pilot study. Neurology. 2006;67:340-342.
25. Agarwal P, Kumar N, Chandra R, et al. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16:527-532.
26. Gilad R, Izkovitz N, Dabby R, et al. Treatment of status
epilepticus and acute repetitive seizures with i.v. valproic acid vs
phenytoin. Acta Neurol Scand. 2008;118:296-300.
27. Zelano J, Kumlien E. Levetiracetam as alternative stage two antiepileptic drug in status epilepticus: a systematic review. Seizure. 2012;21:233-236.
28. Knake S, Gruener J, Hattemer K, et al. Intravenous levetiracetam
in the treatment of benzodiazepine refractory status epilepticus. J Neurol Neurosurg Psychiatry. 2008;79:588-589.
29. Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in
status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;259:645-648.
30. Höfler J, Unterberger I, Dobesberger J, et al. Intravenous lacosamide in status epilepticus and seizure clusters. Epilepsia. 2011;52:e148-e152.
31. Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status
epilepticus: a prospective comparison of diazepam and phenytoin versus
phenobarbital and optional phenytoin. Neurology. 1988;38:202-207.
To comment on this article, contact rdavidson@uspharmacist.com.





