ABSTRACT: The majority of prescription orders written in the United States are filled with generic alternatives. The use of generic medications has saved the U.S. healthcare system trillions of dollars. Although prices for generic medications have been rising recently, generics are still very important to the healthcare system and are significantly more cost-effective when compared with their brand alternatives. Therefore, it is important to understand the generic approval process and to be aware of first generic drug approvals. This information can help pharmacists facilitate care and improve overall outcomes.
US Pharm. 2017;42(6)(Generic Drugs suppl):31-37.
The Hatch-Waxman Act, passed in 1984, permitted the approval of generic drugs as equivalents to their brand-name counterparts. A drug manufacturer can get an expedited review for a generic product through the process of submitting an Abbreviated New Drug Application (ANDA) to the FDA. In order to have an ANDA approved by the FDA, certain criteria regarding the brand-drug patent must be met. These may include: that the patent information was never filed; the patent itself has expired; the patent will expire soon; or the patent was invalid or is not violated by the proposed ANDA.1 If any one of these criteria is met, the FDA can move forward with approving the proposed ANDA.2 The median length of postapproval market exclusivity for the widely used brand products is 12.5 years.3
Once the ANDA is approved, the FDA rewards the first manufacturer to file the ANDA with 180-day generic-drug exclusivity. This exclusivity simply means that for 180 days the marketing rights are limited to that select generic drug and manufacturing company. If there are multiple first-to-file applicants, then the 180-day exclusivity will be shared among the applicants.4 Consequently, during this 180-day exclusivity period the cost of the generic product is still relatively high and comparable to the brand product.
In 2016, the FDA’s office of generic drugs approved 630 ANDAs and approved 73 first generic drugs.2 The FDA defines a first generic drug as the first approval of a generic by the FDA, which permits a manufacturer to market a generic drug in the U.S.5 First generics help reduce the cost of the previous higher-priced brand name–only products, thus enabling more affordable medications and greater access to healthcare. The FDA considers first generics to be a health priority and expedites the review of these applications.2
Eighty-nine percent of prescriptions dispensed in the United States are filled with generic medications, and over the past 10 years, generic medications saved the U.S. healthcare system almost $1.5 trillion dollars.6 Drug prices declined to 55% of the brand-name product price when two generic manufacturers are producing the generic product, 33% with five manufacturers, and 13% with 15 manufacturers.7
Although generic medications help consumers save on their prescription costs, over the last several years prescription-drug prices have been rising. In 2015, an analysis by the healthcare data company Truveris found that overall drug prices have increased by more than 10%. This was well above the U.S. inflation rate for 2015. Additionally, their research concluded that the nation as a whole was in its third year of seeing double-digit increases in drug prices in almost every drug category. Generic-drug prices rose by 2.93% and brand-name drugs increased by 14.77%.8
Several factors are responsible for the rise in generic- drug costs, including merging of drug manufacturers, which has led to decreased competition in the generic- drug market. For example, in 2015, Turing Pharmaceuticals increased the price on pyrimethamine by 5,500%, from $13.50 to $750 a pill.9 Since there was no other manufacturer of this product, Turing owned the market and could set the price. Similar situations have occurred with other generic drugs, like digoxin, isoproterenol, and nitroprusside.10 While lack of competition is the most likely cause of increased prices, shortages of raw materials, a decrease in new generic medications, and FDA regulations are also considered possible contributing factors to the increase in drug prices.8 Although generic drugs have experienced a rise in cost, most are still priced well below drugs that are still available only as brand-name products.
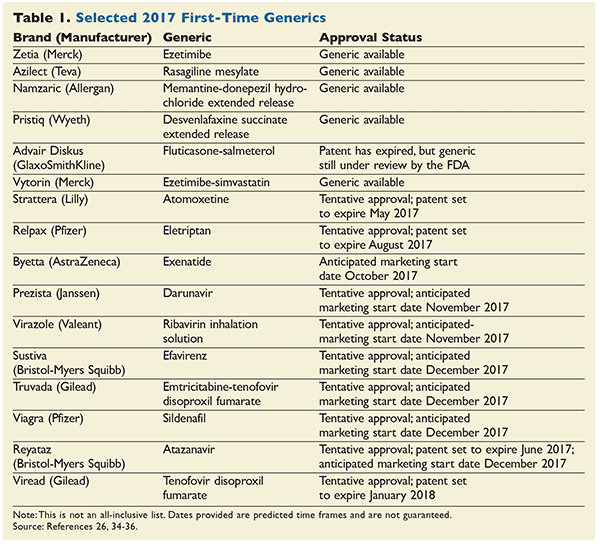
Though the FDA approved a record-breaking number of ANDAs in 2016 and the median time for a brand drug to hold a patent is 12.5 years, drug manufacturers have found ways to extend the life of a patent.3,11 Manufacturers refer to this as product life-cycle management. This involves trying to prevent generic competition and maintain drug exclusivity. Brand-drug manufacturers can achieve this by obtaining additional patents on other aspects of a drug.12,13 Therefore, it is difficult to accurately predict when a generic equivalent will be marketed. Table 1 lists first generics released in the U.S. at the end of 2016, and the predicted first generics for 2017. This article will briefly discuss these new generic medications.
Cardiovascular Agents
Zetia (ezetimibe): Ezetimibe, the generic equivalent for Zetia (ezetimibe), was released at the end of 2016. Ezetimibe is an antilipemic agent used in the treatment of hyperlipidemia. Even though statins are considered first-line treatment of hyperlipidemia, ezetimibe is commonly used in combination with statins. When used in combination, ezetimibe works to inhibit cholesterol absorption and can help reduce the dose of the statin by 12% to 20%. While it seems as though ezetimibe is efficacious, studies have shown that ezetimibe does not affect surrogate endpoints.14
Vytorin (ezetimibe-simvastatin): The generic equivalent for Vytorin (ezetimibe-simvastatin) was released earlier this year. It has taken this generic longer than expected to enter the market. Merck and Company, the manufacturer of Vytorin, underwent litigation with Mylan, the drug manufacturer seeking to produce a generic formulation of ezetimibe-simvastatin. Merck claimed the expiration date of its patent was April 2017, and in February 2013 the court ruled in favor of Merck, granting the patent exclusivity until April 2017. Merck has previously stated that sales of Vytorin are approximately $1.75 billion a year, indicating that a generic's coming to market would greatly impact the manufacturer’s sales.15
Parkinson’s Disease Agent
Azilect (rasagiline mesylate): is an FDA-approved second-generation selective monoamine oxidase-B inhibitor used for the treatment of Parkinson’s disease. Rasagiline has been shown to be efficacious in treating Parkinson’s disease when used alone or in combination with other treatments.16 The average wholesale price (AWP) for a 30-day supply of the brand product Azilect is approximately $833.00.17 The AB-rated generic of Azilect is now available.
Alzheimer’s Disease Agent
Namzaric (memantine-donepezil): The newly approved generic for Namzaric (memantine-donepezil) was released in February 2017. This combination medication is indicated for the treatment of moderate-to-severe Alzheimer’s dementia.18 Combination therapy of an acetylcholinestrase inhibitor (donepezil) and an N-methyl D-aspartate (NMDA) antagonist (memantine) is often used in severe Alzheimer’s dementia. Combining the two products decreases the pill burden for a patient population that takes numerous medications. The AWP for a 30-day supply of Namzaric was $463.00.17
Central Nervous System Agents
Pristiq (desvenlafaxine succinate): The AB-rated generic equivalent for Pristiq (desvenlafaxine succinate) was released in March 2017. Desvenlafaxine is a serotonin-norepinephrine reuptake inhibitor (SNRI) that is approved for treatment of major depressive disorder.19 Another desvenlafaxine extended-release tablet has been on the market since 2013, but this product is not AB-rated to Pristiq; it does not contain the salt form, succinate, and therefore is not an AB-rated therapeutic equivalent. The recently approved desvenlafaxine succinate is the only AB-rated therapeutic equivalent. The AWP for a 30-day supply of Pristiq is $382.44.17
Strattera (atomoxetine): The generic equivalent for Strattera (atomoxetine) has the potential to be approved in 2017. Atomoxetine is a norepinephrine reuptake inhibitor indicated for the treatment of attention-deficit/hyperactivity disorder.20 The ANDA for ato-moxetine has tentative approval, and according to the FDA website, the patent for Strattera is currently set to expire in May 2017. The AWP for Strattera ranges from $474.48 to $556.20.17
Relpax (eletriptan): Relpax (eletriptan) may also be released in 2017. Eletriptan is a triptan drug used to treat acute migraines with and without aura.21 The patent is currently set to expire in August 2017 and several generic manufacturers have tentative approval on their ANDAs. The AWP for the six-tablet pack of Relpax is $374.39.17
Asthma
Advair Diskus (fluticasone-salmeterol): According to the FDA website, the patent for GlaxoSmithKline’s leading product, Advair Diskus (fluticasone-salmeterol), expired August 23, 2016.22 The drug manufacturer Mylan is in the process of bringing a generic version to market. In recent news, Mylan received a complete response letter from the FDA, also known as a bad-news notification, concerning its ANDA. Much of the holdup is likely due to the hard-to-copy diskus technology.23 Advair is indicated for the treatment of asthma and chronic obstructive pulmonary disease (COPD). The approval of a generic product in this category of combination inhaled corticosteroid and long-acting beta agonist will be important for consumers, as none of the products in this class have a generic alternative. Depending on the strength of the product, an Advair Diskus for a 30-day supply can run from approximately $167 to $272 dollars.17
Anti-diabetic Agent
Byetta (exenatide): The CDC states that within the U.S., approximately 29.1 million people have diabetes mellitus. Of those, only 21 million have actually been diagnosed, and their number is predicted to rise, as it is estimated that 8.1 million people are currently undiagnosed.24 Diabetes not only has a major impact on a patient’s life, but is also very expensive to manage. In 2013, it was believed that approximately $176 billion of U.S. healthcare costs went toward direct medical costs associated with diabetes and that the disease cost $69 billion in reduced productivity.25 Patients with diabetes often require more than one antidiabetic medication to control their blood glucose. Thus, it is important to offer generic drugs that can provide a cost savings. In 2017, the patent for Byetta (exenatide) is due to expire; a generic will be marketed by Teva in October 2017.26 Exenatide is a glucagon-like peptide agonist (GLP-1) indicated for the treatment of type 2-diabetes. The AWP for Byetta (pen) for a 30-day supply is $801.94.17 Therefore, the approval of the new generic will allow for significant cost savings.
Phosphodiesterase-5 Inhibitor
The Massachusetts Male Aging Study found that 52% of men age 40 to 70 years experience erectile dysfunction (ED).27 While the incidence of ED has been shown to increase with age, most people do not seek medical attention.28 Though increased age may lead to ED, this disorder may also be caused by certain medical conditions such as hypertension, diabetes, or psychiatric disorders.29 Certain medications have also been shown to increase the incidence of ED, including beta blockers.30
Viagra (sildenafil): A study by the Johns Hopkins Bloomberg School of Public Health found that more than 18 million men aged 20 and older are affected by erectile dysfunction (ED) in the U.S.31 The road to making the ED drug Viagra (sildenafil) generic has not been smooth. Viagra has become one of the most popular brand-name medications for the treatment of ED in the past few years. Originally, Viagra’s patent was to expire in March of 2012. In March 2010, Pfizer sued Teva for patent infringement and Pfizer won the case. It was declared that the patent would stand until 2020. Pfizer later reported that Teva had agreed to pay it an undisclosed amount, and granted the generic company a license to produce a generic version of Viagra in December 2017. Teva will be the only manufacturer allowed to produce the generic product until 2020.32 The AWP for a 30-count bottle of Viagra is $1,962.32.17
Antiretroviral Agents
Reyataz (atazanavir) and Others: The treatment of HIV is not only a lifelong process, it is a costly one. Studies show that the sooner a patient is initiated on antiretroviral therapy, the better the outcome. However, patient adherence to these medications is generally low, owing to the expense and the burden to the patient. In many instances, a patient trying to obtain antiretroviral therapy may run into cost sharing (the patient is responsible for some of the cost of the medication), prior authorizations (especially with newer agents), or the lack of availability of a generic alternative. The cost per month for an antiretroviral medication can range from several hundred to thousands of dollars for one medication. As most HIV regimens require a combination of medications, the cost and burden to the patient quickly begins to increase.33 In 2017 and 2018, five antiretroviral medications may become available as generics. These include Reyataz (atazanavir), Sustiva (efavirenz), Viread (tenofovir disoproxil fumarate tablet), Prezista (darunavir tablet), and Truvada (emtricitabine-tenofovir disoproxil fumarate tablet). The AWP of these products for a 30-day supply ranges from approximately $1,100 to $1,800.17
Conclusion
While brand-name medications require exclusivity, this can in some instances increase the burden of cost on patients. Generics can help to decrease this burden, but it is a delicate balance. The generic-drug approval process is multi-factorial and roadblocks can be created, ultimately leading to the delay of getting a generic to market. Pharmacists should be aware of the generic drug approval process as well as upcoming first generic drug approvals prior to launch.
REFERENCES
1. Boehm G, Yao L, Han L, Zheng Q. Development of the generic drug industry in the US after the Hatch Waxman Act of 1984. Acta Pharmaceutica Sinica B. 2013;3:297-311.
2. FDA. Office of Generic Drugs. Annual report for 2016. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm542710.htm. Accessed March 3, 2017.
3. Wang B, Liu J, Kesselheim AS. Variations in time of market exclusivity among top-selling prescription drugs in the United States. JAMA Intern Med. 2015;175:635-637.
4. FDA. FDA/CDER SBIA Chronicles. Patents and exclusivity. May 19, 2015. www.fda.gov/downloads/drugs/developmentapprovalprocess/smallbusinessassistance/ucm447307.pdf. Accessed February 18, 2017.
5. FDA. Drugs. First generic drug approvals. Updated May 4, 2017. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ANDAGenericDrugApprovals/default.htm. Accessed May 9, 2017.
6. Generic Pharmaceutical Association. Generic drug savings in the U.S. 7th annual edition: 2015. www.gphaonline.org/media/wysiwyg/PDF/GPhA_Savings_Report_2015.pdf. . Accessed February 2, 2017.
7. FDA. Generic competition and drug prices. www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm129385.htm. Accessed March 15, 2017.
8. Dennis B. Prescription drug prices jumped more than 10 percent in 2015, analysis finds. Washington Post. January 11, 2016. www.washingtonpost.com/news/to-your-health/wp/2016/01/11/prescription-drug-prices-jumped-more-than-10-percent-in-2015/?utm_term=.cef5eb4a84ee. Accessed February 19, 2017.
9. Pollack A. Drug goes from $13.50 a tablet to $750, overnight. New York Times. September 20, 2015. www.nytimes.com/2015/09/21/business/a-huge-overnight-increase-in-a-drugs-price-raises-protests.html?_r=0. Accessed February 28, 2017.
10. O’Brien E. Why drug prices remain insanely high and 6 things you can do to save. Marketwatch. September 21, 2015. www.marketwatch.com/story/six-tips-for-fighting-rising-prescription-drug-costs-2015-09-15. Accessed March 5, 2017.
11. Grabowski H, Long G, Mortimer R, Boyo A. Updated trends in US brand-name and generic drug competition. J of Med Econ. 2016;19:836-844.
12. Kapczynski A, Park C, Sampat B. Polymorphs and prodrugs and salts (oh my!): an empirical analysis of “secondary” pharmaceutical patents. PLoS One; 2012;7:e49470.
13. Silverman E. Reckitt’s suboxone strategy is really about patients or profits? Forbes. October 12, 2012. www.forbes.com/sites/edsilverman/2012/10/12/reckitts-suboxone-strategy-is-really-about-patients-or-profits/#24d6e1e86c3f. Accessed March 5, 2017.
14. Robinson JG, Davidson MH. Combination therapy with ezetimibe and simvastatin to achieve aggressive LDL reduction. Expert Rev Cardiovasc Ther. 2006;4:461-476.
15. Bartz D, Peirson R. Appeals court upholds patent on Merk’s Vytorin. February 7, 2013. Reuters. www.reuters.com/article/us-usa-patents-merck-idUSBRE9160XG20130207. Accessed February 19, 2017.
16. Chang Y, Wang L-B, Li D,et al. Efficacy of rasagiline for the treatment of Parkinson’s disease: an updated meta-analysis. Ann Med. 2017:1-14.
17. Red Book Online [database online]. Greenwood Village CO: Truven Health Analytics. www.micromedexsolutions.com. Updated 2017. Accessed March 30, 2017.
18. Namzaric (memantine-donepezil) package insert. Irvine, CA: Allergan; 2016. www.allergan.com/assets/pdf/namzaric_pi/. Accessed March 5, 2017.
19. Pristiq (desvenlafaxine succinate) package insert. Philadelphia, PA: Pfizer; July 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2012/021992s030lbl.pdf. Accessed March 12, 2017.
20. Strattera (atomoxetine) package insert. Indianapolis, IN: Eli Lilly and Company; April 2015. http://pi.lilly.com/us/strattera-pi.pdf. Accessed March 12, 2017.
21. Relpax (eletriptan) package insert. New York, NY: Pfizer; November 2013. http://labeling.pfizer.com/ShowLabeling.aspx?id=621. Accessed March 8, 2017.
22. Advair Diskus (fluticasone-salmeterol) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2008. www.accessdata.fda.gov/drugsatfda_docs/label/2008/021077s029lbl.pdf. Accessed March 28, 2017.
23. Helfand C. Mylan’s generic Advair gets stiff-armed by FDA. What does this mean for GSK? March 29, 2017. www.fiercepharma.com/pharma/where-fda-s-decision-mylan-s-generic-advair-and-what-could-it-mean-for-glaxo. Accessed March 29, 2017.
24. CDC. 2017 National diabetes statistics report. May 15, 2015. www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Accessed February 19, 2017.
25. American Diabetes Association. Statistics about diabetes. May 18, 2015. www.diabetes.org/diabetes-basics/statistics/?referrer=https://www.google.com/. Accessed February 18, 2017.
26. Corporate Pharmacy Services. Upcoming generic drugs. www.corporatepharmacy.com/page/upcoming_generic_drugs. Accessed March 5, 2017.
27. NIH Consensus Conference. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83-90.
28. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
29. Albersen M, Orabi H, Lue TF. Evaluation and treatment of erectile dysfunction in the aging male: a mini-review. Gerontology. 2012;58:3-14.
30. Berookhim BM, Bar-Chama N. Medical implications of erectile dysfunction. Med Clin North Am. 2011;95:213-221.
31. Selvin E, Burnett AL, Platz E. 18 million men in the United States affected by erectile dysfunction. Johns Hopkins Bloomberg School of Public Health. February 1, 2007. www.jhsph.edu/news/news-releases/2007/selvin-erectile-dysfunction.html. Accessed February 18, 2017.
32. Access Rx. Viagra patent expiration date extended to 2020. www.accessrx.com/research/viagra-patent-expires. Accessed February 20, 2017.
33. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infectedadults and adolescents. July 14, 2016. Department of Health and Human Services. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed February 25, 2017.
34. U.S. National Library of Medicine. Daily Med database. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7195b977-6c91-4fed-837d-9b146f051ce8. Accessed March 5, 2017.
35. FDA. Drugs@FDA: FDA approved drug products. www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed March 5, 2017
36. Therapeutic Research Center. PL Detail-Document #330146: Anticipated availability of first-time generics. Pharmacist’s Letter/Prescriber’s Letter. January 2017. http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=faculty&s=PL&pt=6&fpt=56&dd=330146&pb=PL&searchid=60924288. Accessed February 10, 2017.
To comment on this article, contact rdavidson@uspharmacist.com.






