US
Pharm. 2006;31(1)(Oncology suppl):3-15.
Fungal infections are a major
cause of morbidity and mortality in immunocompromised patients. Filamentous
mold and yeast-like fungi are ubiquitous organisms found worldwide in many
different media. The Candida species are the most common cause of
fungal infections. However, epidemiologic shifts have begun to occur, most
likely due to the prophylactic and empiric use of antifungal agents. Emerging
fungal pathogens, such as Aspergillus, Fusarium, and
Zygomycetes, are changing the clinical spectrum of fungal diagnoses.
Pharmacists have more choices than ever before to aid in the treatment of
these highly morbid and lethal infections.
Pathogens
Fungi are
opportunistic pathogens that take advantage of immune system defects and are
capable of causing disease in many sites and settings. Common fungal
infections are cutaneous (including nails), disseminated fungemia, pneumonia,
peritonitis, osteomyelitis, and endocarditis. Fungal infections may also occur
following various types of traumatic injuries.
General risk factors for
invasive fungal infections are exposure to pathogens, an impaired immune
system, and fungal spores. The presence of a colonized environment, partnered
with a disruption in a physiologic barrier, potentiates the risk of an
invasive fungal infection in an immunologically impaired host, such as a
patient infected with HIV, someone taking chronic systemic steroids, or a
transplant recipient. In addition, contaminated implanted devices (e.g.,
catheters, prostheses), external devices (e.g., contact lenses), and community
reservoirs (e.g., hand lotion, pepper shakers) have all been implicated as
sources of fungal outbreaks.1
The Candida species are
particularly hard to eradicate from infections that involve implanted foreign
bodies because they have been shown to create a protective barrier over
colonies as they are established. The exopolymeric nature of these protective
biofilms limits the access of antifungal agents to the pathogens, decreasing
the possibility of a cure when the foreign body still resides within the
patient. The filtering effect of the biofilm, along with the varying fungal
colony growth rates and the ability to excrete antifungals from the colony
within the film, may be key factors in the persistence of candidal infections,
despite adequate antifungal levels and susceptible organisms.2
Candida albicans
continues to be the most frequent cause of invasive fungal infections in most
patient populations. However, prophylaxis and the widespread use of antifungal
agents as empiric therapy for neutropenic fever have led to a shift in the
epidemiology of invasive Candida infections. Infections with species
other than C. albicans (Candida glabrata, Candida parapsilosis
, Candida tropicalis, Candida krusei, and Candida lusitaniae
) are becoming more prevalent. Fluconazole prophylaxis selects for these
emerging species and as a result, they are more likely to be resistant to
triazole antifungal agents. Due to susceptibility variations between species,
species identification and susceptibility testing have become important tools.
3
The second most common fungal
pathogen to cause invasive fungal disease is Aspergillus. Found
worldwide, Aspergillus is able to thrive in almost every environment.
The organism is found primarily in soil but is also commonly isolated from
water, food, and air. The usual route of infection for invasive aspergillosis
is via inhalation of conidia (asexual spores). As a result, the lung is the
most common location of invasive infection. The sinuses, central nervous
system, and skin are also areas that can become infected. Clinically, the most
common species to cause infection are Aspergillus fumigatus,
Aspergillus flavus, Aspergillus terreus, and Aspergillus niger
. Despite the availability of antifungal agents to treat infections caused by
Aspergillus, the morbidity and mortality of invasive aspergillosis remains
high.4
Although still rare,
infections caused by the mold pathogens Fusarium and Zygomycetes
are increasingly prominent.5,6 Fusarium can cause a wide
variety of fungal infections. Resistance to antifungal agents is common;
therefore, Fusarium infections are highly lethal. However, there have
been recent reports of successful treatment with voriconazole.7
Zygomycosis (commonly called mucormycosis) is an extremely aggressive
fungal infection that causes angioinvasive disease.8 It typically
involves the sinuses and spreads rapidly to the brain, resulting in a very
high mortality rate. Cutaneous disease is less common but is associated with a
better outcome. Unfortunately, amphotericin B products are the only antifungal
agents currently available on the market with adequate coverage of species
within this class.
Antifungal Therapy
Diagnosing invasive
fungal infections early, reliably, and definitively continues to be a major
challenge to practitioners. As a result, empiric therapy is generally
initiated in response to a persistent fever in a vulnerable patient.9
Suspicion for a fungal infection is increased when other types of infections,
such as viral or bacterial, have been ruled out and the patient is known to
have fungal colonization or suspicious radiographic findings. Through the
correlation of known host factors, microbiologic knowledge, and clinical
input, a probable fungal infection can be imputed. This is considered
appropriate criteria for beginning empiric antifungal therapy aimed at the
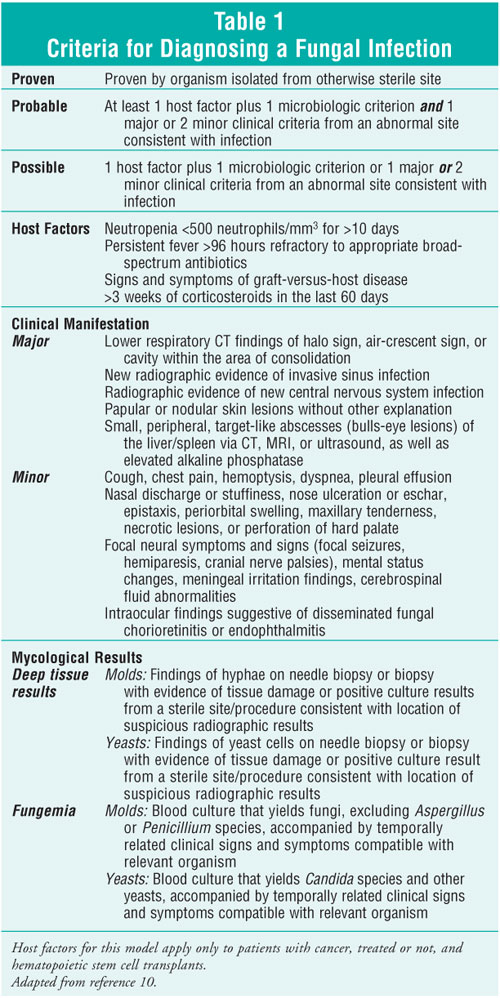
most likely pathogen in that patient population.10 One example of
such a clinical system, validated in cancer and hematopoietic stem cell
transplant (HSCT) patients, is found in table 1. Empiric therapy is generally
begun with a single agent targeted at the most common pathogen. For Candida
, this is usually a triazole antifungal, such as fluconazole. Fluconazole is
still considered the agent of choice for C. albicans. However, if
another Candida species is suspected, an echinocandin should be
selected for its broader spectrum of activity. If a mold is suspected, an
amphotericin product is generally chosen empirically. However, depending on
the certainty of the diagnosis and each patient's specific clinical situation,
an extended-spectrum triazole (e.g., voriconazole) or an echinocandin (e.g.,
caspofungin), both of which have anti-Aspergillus activity, may be
chosen instead. These newer agents are approved for the treatment of
Aspergillus but have variable levels of activity against other types of
filamentous fungi, such as Fusarium and Zygomycetes.
Combination antifungal
therapy has become an area of interest due to the high rates of morbidity and
mortality associated with fungal infections. This tactic was recognized as an
important tool in the treatment of cryptococcal meningitis when flucytosine
was combined with amphotericin B. However, the clinical use of combination
therapy for either established fungal infections or drug-resistant species had
been slow to develop due to in vitro reports of decreased efficacy and
possible antagonism when amphotericin and azoles were used in combination.
Antagonism between these two agents is possible because of their mechanisms of
action. (Azoles block ergosterol synthesis, whereas amphotericin causes
membrane damage by binding to ergosterol.) Yet, with the advent of
echinocandins and extended-spectrum triazoles, combination antifungal therapy
is an area where research has developed dramatically. Echinocandins inhibit
the synthesis of (1,3)-beta-D-glucan (a component of the fungal cell wall),
therefore targeting a different cellular site than azoles or amphotericin. To
improve cure rates against various fungal pathogens (primarily Aspergillus
), combinations of an echinocandin and a triazole or amphotericin B are being
investigated. Good outcomes with combination therapy involving in vitro
studies, animal data, and case reports have been published. In fact, triple
therapy has been used in at least one case report.11 An
observational study evaluating the use of voriconazole and caspofungin as
salvage therapy for invasive aspergillosis against a historical group that
received voriconazole alone reported promising effects of combination therapy.
12 However, comparative clinical data are limited, and the benefit of
combination antifungal therapy still remains uncertain.
Polyenes:
Polyenes act by binding to ergosterol within the fungal cell wall, creating
pores that increase permeability and cause leakage of fungal cell contents.
Amphotericin B is the only systemic agent to belong to the polyene class of
antifungals. Four amphotericin B formulations are currently available on the
market. For more than 40 years, conventional amphotericin B deoxycholate
(Fungizone) has been the standard therapy for invasive fungal infections.
Three lipid-based formulations are also available: amphotericin B colloidal
dispersion (Amphotec), amphotericin B lipid-complex (Abelcet), and liposomal
amphotericin B (Ambisome). All four formulations have excellent activity
against a wide range of fungal pathogens, and resistance to these agents is
rare. The major advantages of the lipid preparations are that they achieve
higher concentrations in the reticuloendothelial organs (liver, lung, and
spleen), they have fewer infusion-related reactions, and they are less
nephrotoxic than conventional amphotericin B. However, superior clinical
efficacy over conventional amphotericin B has not been established in clinical
trials, and the lipid formulations are significantly more expensive in
comparison.
It is well known that
amphotericin B is associated with significant adverse events. Acute
infusion-related reactions, such as fever, chills, rigors, and hypotension,
are most common with the conventional formulation. However, these reactions
can also occur with lipid formulations. The reactions are most frequent during
the initial infusions of amphotericin B and often diminish with subsequent
infusions. Premedication with antipyretics, antihistamines, and
corticosteroids will usually lessen these effects. Amphotericin B causes a
dose-dependent decrease in the glomerular filtration rate that is
dose-limiting. Permanent loss of renal function is thought to be related to
the cumulative total dose, rather than the level of temporary azotemia. Sodium
loading with 500 to 1,000 mL of normal saline prior to each infusion is
believed to lessen the nephrotoxic effects, but its exact effects are
uncertain.13 Amphotericin B can also cause significant electrolyte
abnormalities (hypomagnesemia and hypokalemia). These effects occur in the
majority of patients within the first week of therapy. Electrolytes should be
monitored daily and replaced as needed.
Doses of amphotericin B
deoxycholate for the treatment of invasive fungal infections range from 0.5 to
1.5 mg/kg/day. Higher doses (1 to 1.5 mg/kg/day) are usually utilized for
patients with invasive aspergillosis or mucormycoses. Although the incidence
of acute hypersensitivity reactions is rare, and many clinicians feel that it
is unnecessary, a test dose of 1 mg can be administered. Initial doses of the
lipid preparations range from 2.5 to 5 mg/kg/day. Dosage adjustments for renal
or hepatic dysfunction are unnecessary for any of the formulations, but renal
insufficiency often limits its use. Drug interactions with amphotericin
products are minimal. However, concomitant administration with other
nephrotoxic drugs (e.g., aminoglycosides and cyclosporine) warrants caution.
Triazoles:
Triazole antifungals agents exhibit their effects by blocking the synthesis of
ergosterol, a component of the fungal cell wall. As a result, the integrity of
the cell wall is compromised, and increased permeability leads to cell lysis
and subsequent cell death. Fluconazole, itraconazole, and voriconazole are
available in both oral and intravenous formulations. The less toxic effects to
the kidneys make triazoles a more desirable choice than amphotericin B.
However, due to their effects on the liver enzyme systems, drug interactions
are common with these agents. The susceptibility patterns of triazoles vary
for different species of yeast and molds.
Fluconazole has excellent
activity against most species of Candida. However, the use of this
agent for prophylaxis has resulted in increased resistance among non-
albicans species. Fluconazole is not active against Aspergillus,
Fusarium, or Zygomycetes. The dosage of fluconazole varies greatly
depending on the infection site and species of Candida. Normal doses of
fluconazole vary from 100 mg daily for oropharyngeal infections to 800 mg
daily for serious infections. Oral formulations are highly bioavailable; thus,
oral and intravenous dosages are the same. Renal dysfunction requires dosage
adjustment. Adverse effects of fluconazole include headache, gastrointestinal
disturbances, rash, and hepatic dysfunction. Fluconazole is excreted primarily
in the urine as unchanged drug. However, it does inhibit the liver enzyme
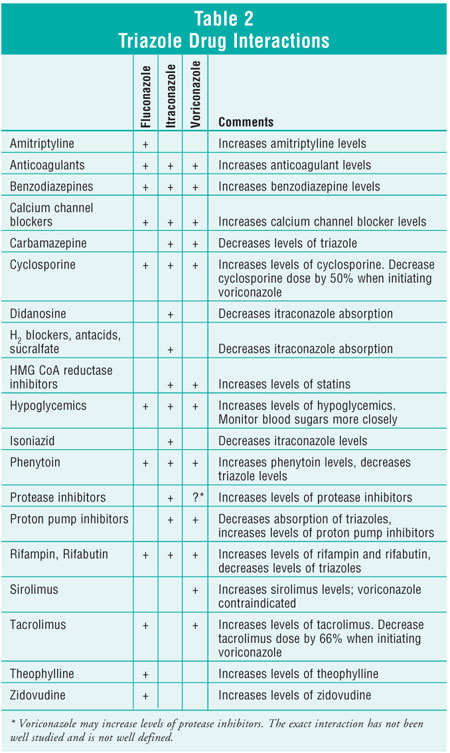
systems cytochrome P450 (CYP) 2C8/9, CYP2C19, and CYP3A4. Drug interactions
are common but are less significant than other agents within this class. See
table 2 for drug interactions.
In addition to activity against
Candida, itraconazole also has activity against Aspergillus. Oral
formulations are poorly bioavailable. Oral capsules should be taken with food,
whereas the oral solution should be taken on an empty stomach. Oral
itraconazole should not be administered with antacids or other medications
that affect gastric pH because coadministration can decrease absorption
significantly. The appropriate dosage of intravenous itraconazole for
Aspergillus is 200 mg twice daily for four doses, followed by 200 mg
daily. Intravenous itraconazole should be avoided in patients with creatinine
clearances of less than 30 mL per minute due to reduced clearance of the
intravenous vehicle hydroxypropyl-beta-cyclodextrin. Oral itraconazole should
be taken as a 200-mg dose three times daily for three days, followed by 200 mg
twice daily. The oral solution has an undesirable taste, which is poorly
masked despite attempts at flavor enhancement. The most common adverse effect
is dose-related gastrointestinal upset. Other adverse effects include
headache, rash, and elevated liver function tests. Itraconazole is a substrate
and inhibitor of CYP3A4. As a result, there are many significant drug
interactions. See table 2.
Exhibiting activity similar to
fluconazole against Candida, voriconazole also has added coverage of
Aspergillus, Fusarium, and several other yeasts and molds.
Voriconazole is generally well tolerated but has significantly more side
effects and drug interactions than fluconazole. The most common side effect is
visual disturbances, occurring in approximately 30% of patients. Visual
disturbances (e.g., color changes, blurry vision, photophobia, decreased
visual acuity, rare hallucinations) appear in approximately one third of
patients within the first week of therapy. These visual effects are reversible
upon discontinuation; however, voriconazole is rarely discontinued secondary
to these changes since the disturbances typically diminish or disappear during
the course of therapy.14 Other less typical side effects are rash,
elevated liver function tests, headache, and gastrointestinal irritation.
Voriconazole is metabolized via CYP2C9, CYP3A4, and CYP2C19 isoenzymes in the
liver. It also inhibits each of these enzymes. Consequently, the potential for
drug interactions is extremely high (see table 2). Therapy with voriconazole
should be initiated with two intravenous loading doses of 6 mg/kg every 12
hours. Maintenance therapy is 4 mg/kg intravenously every 12 hours or 200 mg
orally twice daily for patients who weigh more than 40 kg. The dosage for
patients who weigh less than 40 kg is 100 mg orally twice daily. Oral
bioavailability exceeds 90%, and patients can be easily transitioned to oral
therapy. Maintenance doses of voriconazole should be decreased by 50% in
patients with mild to moderate hepatic dysfunction (Child-Pugh classes A and
B). Voriconazole demonstrates nonlinear pharmacokinetics and considerable
interpatient variability with regard to metabolism. As a result, some
institutions are monitoring voriconazole drug levels.15 However,
considering the uncertain availability of testing, this practice has not yet
become routine, and the clinical significance still remains to be determined.
Intravenous voriconazole should be avoided in patients with creatinine
clearances of less than 50 mL per minute, secondary to accumulation of the
intravenous vehicle sulfobutyl ether beta-cyclodextrin sodium.14
Echinocandins:
The echinocandins caspofungin and micafungin exhibit their antifungal activity
by inhibiting the synthesis of (1,3)-beta-D-glucan (a component of the fungal
cell wall), resulting in reduced fungal cell wall integrity and subsequent
cell death. Both agents are available only as intravenous formulations and
have excellent activity against the majority of Candida species,
including those resistant to triazoles. Echinocandins also have activity
against Aspergillus but are only effective against actively growing and
dividing forms. The activity of echinocandins against other fungal pathogens
is variable.
Caspofungin is generally well
tolerated. Adverse effects are headache, increased liver function tests,
infusion site reactions, and other symptoms related to histamine release.
Caspofungin undergoes hepatic metabolism via spontaneous peptide hydrolysis
and N-acetylation. Metabolites are inactive and excreted primarily via the
urine and feces. Caspofungin is not removed by dialysis, and dose adjustments
for renal insufficiency are unnecessary. However, plasma concentrations
increase with hepatic insufficiency. An initial dose of 70 mg is administered,
followed by a 50-mg daily dose in patients with normal hepatic function.
Patients with moderate hepatic insufficiency (Child-Pugh score 7–9) should
receive the initial 70-mg loading dose, followed by a reduced daily dose of 35
mg. Dosage recommendations are not made for more severe hepatic dysfunction.
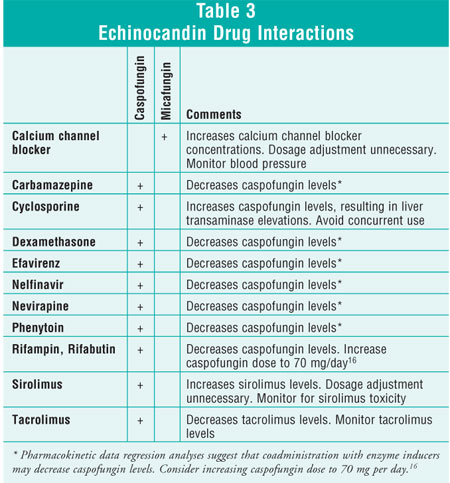
Although caspofungin does not appear to inhibit P-glycoprotein or hepatic CYP
enzymes, several drug interactions do exist (see table 3).
Approved in March 2005, micafungin
is currently indicated for the treatment of patients with esophageal
candidiasis and as prophylaxis of Candida infections in patients
undergoing HSCT.17 The spectrum of antifungal activity is similar
to that of caspofungin. The recommended dose for the prophylaxis of Candida
infections in HSCT patients is 50 mg/day. The treatment of esophageal
candidiasis requires 150 mg per day. Dose adjustment is not necessary for
renal or hepatic impairment. Micafungin has an adverse effect and safety
profile similar to that of caspofungin. However, micafungin is metabolized by
catechol O-methyltransferase, does not affect P-glycoprotein, and appears to
only minimally affect the CYP enzyme systems. As a result, significant drug
interactions are minimal.
Pyrimidine:
Flucytosine is the only agent in the pyrimidine class of antifungals. The
antifungal effects of flucytosine are exerted by its conversion to
5-fluorouracil within the fungal cell and interference with fungal RNA and
protein synthesis. It has activity against Candida, Cryptococcus
, and several molds. However, resistance develops quickly when flucytosine is
used as the sole antifungal agent; therefore, it is used usually only in
combination with other agents. Its use is also significantly hampered by
frequent and serious adverse effects such as bone marrow suppression, hepatic
dysfunction, renal dysfunction, rash, and gastrointestinal disturbances.
Flucytosine is given orally at doses of 50 to 150 mg/kg/day divided every six
hours and requires dosage adjustment for renal dysfunction. Therapy often
requires monitoring serum flucytosine concentrations to avoid the
dose-dependent bone marrow suppression. Since this agent is primarily
eliminated renally as an unchanged drug, interactions are minimal.
Investigational Agents:
Several investigational antifungal agents may soon be available. Posaconazole
and ravuconazole are triazole antifungals that are currently in various stages
of development. Posaconazole has demonstrated in vitro activity against
Zygomycetes.18 Anidulafungin is an investigational echinocandin
that is now being evaluated in clinical trials.
Pharmacist's Role
Despite recent
advances in antifungal therapy, fungal infections continue to be a significant
threat to immunocompromised patients. It is important for the pharmacist to
know the common fungal pathogens that are seen in various patient groups. By
assessing patients' risk factors, culture results, radiographic information,
organ function parameters, clinical symptoms, and comorbidities, pharmacists
can guide both empiric and pathogen-directed therapy. Depending on the
pathogen involved, significantly decreased renal function makes amphotericin
products less appealing and increases the suitability of a triazole or
echinocandin. Diminished hepatic function may require dose adjustments of some
antifungals or lead the medication selection toward an agent that is
metabolized via another route. Other medical conditions that require a patient
to take interacting medications will also rule out or further guide the
selection when multiple drugs have the potential to treat a patient's
infection. An individual patient's drug of choice is rarely determined by a
single therapeutic factor. It is frequently the pharmacist who is best able to
sort through the many variables to choose the optimal medication.
REFERENCES
1. Safdar A.
Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid
response to treatment with caspofungin and itraconazole. Clin Infect Dis
. 2002;34:1415-1417.
2. Douglas LJ. Candida
biofilms and their role in infection. Trends Microbiol. 2003;11:30-36.
3. Pappas PG, Rex JH,
Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis
. 2004;38:161-189.
4. Lin SJ, Schranz J,
Teutsch SM. Aspergillosis case-fatality rate: systematic review of the
literature. Clin Infect Dis. 2001;32:358-366.
5. Nucci M, Marr KA,
Qeuiroz-Telles F, et al. Fusarium infection in hematopoietic stem cell
transplant recipients. Clin Infect Dis. 2004;38:1237-1242.
6. Kauffman CA.
Zygomycosis: reemergence of an old pathogen. Clin Infect Dis.
2004;39:588-590.
7. Consigny S, Dhedin
N, Datry A, et al. Successful voriconazole treatment of disseminated fusarium
infection in an immunocompromised patient. Clin Infect Dis.
2003;37:311-313.
8. Walsh TJ, Groll A,
Hiemenz J, et al. Infections due to emerging and uncommon medically important
fungal pathogens. Clin Microbiol Infect. 2004;10(suppl 1):48-66.
9. Hughes WT, Armstrong
D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in
neutropenic patients with cancer. Clin Infect Dis. 2002;34:730-751.
10. Ascioglu S, Rex JH,
de Pauw B, et al. Defining opportunistic invasive fungal infections in
immunocompromised patients with cancer and hematopoietic stem cell
transplants: an international consensus. Clin Infect Dis. 2002;34:7-14.
11. Sims-McCallum RP.
Triple antifungal therapy for the treatment of invasive Aspergillus in a
neutropenic pediatric patient. Am J Health-Syst Pharm.
2003;60:2352-2356.
12. Marr K, Boeckh M,
Carter R, et al. Combination antifungal therapy for invasive aspergillosis.
Clin Infect Dis. 2004;39:797-802.
13. Godwin S, Cleary J,
Walawander C, et al. Pretreatment regimens for adverse events related to
infusion of amphotericin B. Clin Infect Dis. 1995;20:755-761.
14. Johnson L, Kauffman
C. Voriconazole: a new triazole antifungal agent. Clin Infect Dis.
2003;36:630-637.
15. Trifilio S, Ortiz
R, Pennick G, et al. Voriconazole therapeutic drug monitoring in allogeneic
hematopoietic stem cell transplant recipients. Bone Marrow Transplant.
2005;35:509-513.
16. Cancidas package
insert. Whitehouse Station, NJ: Merck & Co, Inc. 2001.
17. Mycamine package
insert. Deerfield, IL: Astellas Pharm US, Inc. April 2005.
18. Sun QN, Najvar LK,
Bocanegra A, et al. In vivo activity of posaconazole against Mucor spp. in an
immunosuppressed-mouse model. Antimicrob Agents Chemother.
2002;46:2310-2312.
To comment on this article,
contact
editor@uspharmacist.com.






