US Pharm. 2007;32(12):70-73.
While the overall incidence of lead
poisoning has decreased, it is still prevalent. News of toy manufacturers
using lead paint has drawn attention to a threat that never really went away.
In the United States today, 1.6% of children have toxic blood levels, compared
to 4.4% in the early 1990s.1 Approximately 10.1 in 100,000 of
adults were estimated to be suffering from lead toxicity in 2002.2,3
Lead is the most common neurotoxin in the environment.4 Current
standards define a lead blood level of 10 mcg/dL in the as being toxic in
children.5 In adults, a level of 25 mcg/dL is considered toxic.
2,3 Any level of lead can have toxic manifestations, and all health care
practitioners should become familiar with the signs, symptoms, and treatment
of lead poisoning.
Adults and children become
exposed to lead in different ways. A toxic lead load may be passed through the
umbilical cord in the prenatal period.6 Children become exposed
through inhalation of lead dust, a reflection of environmental lead from
street dust, ground soil, and old house paint.7 Children also
become exposed directly through the soil, drinking contaminated water, or
eating lead-based paints.8,9 Heavily carpeted homes may trap lead
dust, exposing children to higher levels of the metal in buildings already
contaminated.7 Either by direct ingestion or through its presence
in lead dust, lead-based paint is the most common cause of toxic blood levels
in children.8,9 Lead-based paint was phased out in the 1970s,
and children living in structures built after that time are at lower risk.
10 Adults are at risk if they work in lead smelting, lead refining,
battery manufacturing, automobile repair, or manufacturing of lead-based
products.11,12
Clinical Presentation and
Diagnosis
Lead manifests
itself in virtually every organ system and may present in a variety of ways.
13-15 Age plays a role in the specific manifestation of lead exposure,
and adults and children often presenting in different ways even when the same
organ systems are involved.16 An individual with toxic lead levels
may present with any or none of the classic signs or symptoms.
The blood lead level (BLL) is
a direct measurement of lead. Lead can be measured in the long bones
indirectly as lead lines on plain film radiographs.6,8,12,14,17 A
physical exam may reveal a blue line, known as Burton's line,
where teeth and gums meet, caused by a chemical interaction between lead and
the sulfur ions released by oral bacteria.15 A blood smear may show
basophilic stippling as a result of the clustering of ribosomes.16,17
In addition, urine may have increased concentration of aminolevulinic acid.
18
In children, the biggest
concerns associated with long-term lead exposure are cognitive and
neurobehavioral abnormalities. There is a correlation between rising BLLs and
diminished achievement on mental aptitude tests, with symptoms possible even
at nontoxic levels.5,19 Consequences range from mild decreases in
IQ or manifestations of attention-deficit hyperactivity disorder to complete
arrest of neurobehavioral development and toxic encephalopathy.5,16,19
Adults can also suffer cognitive and neurobehavioral consequences, though
these usually manifest as a longitudinal decline at lower levels.20
This decline can be the result of a single, acute exposure, with subsequent
lead buildup in the body, or a low-level, chronic exposure.20,21
Individuals exposed to lead
are also at risk for a myriad of noncognitive neurologic symptoms. These
include fatigue, tremors, parasthesias, headache, ataxia, distal neuropathy,
vertigo, delusions, hallucinations, convulsions, hearing loss, insomnia, and
muscle weakness.12,14,16,17 Lead toxicity is also associated with a
variety of ocular manifestations, including cataracts.22
Lead can also lead to fatigue
by causing anemia. In adults, this is a microcytic microchromic anemia.
16,17 All ages may present with hemolytic anemia at higher levels of
exposure.12,16 Microcytic anemia due to lead is less common in
children.23
Hypertension and renal disease
are associated with lead toxicity. Hypertension is associated with both acute
and chronic lead exposure but does not follow a linear progression
in severity with increasing lead levels.24,25 Individuals with
elevated lead levels may present with lead nephropathy, characterized by
proximal tubule dysfunction.16 There is also a risk of chronic
kidney disease and subsequent end-stage renal disease.16,18,26
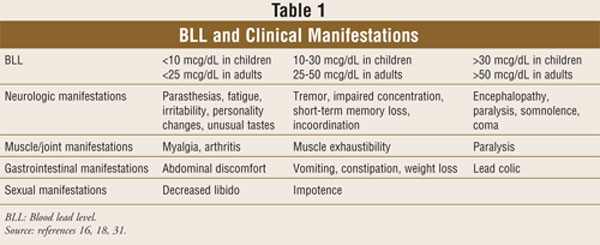
Various gastrointestinal
symptoms are associated with lead toxicity. At low levels, lead may cause
vague, nonspecific abdominal symptoms, such as generalized discomfort,
constipation, or vomiting. Recurrent, severe abdominal pain as a result of
lead exposure is known as lead colicand is associated with
significantly increased blood lead levels.17,18
Lead may also lead to
decreased libido or impotence.12,16 Joint and endocrine complaints
are also possible.12,16 Virtually any presenting complaint can be a
manifestation of lead toxicity.
Treatment
The cognitive and
behavioral effects of lead toxicity are not reversible; therefore, prevention
is crucial.27-29 The management of lead toxicity in children is
based on BLL. Severe lead intoxication is defined by a venous BLL of ?70
mcg/dL or having signs and symptoms of encephalopathy.27-29 This is
a medical emergency and is treated by parenteral chelating agents dimercaprol
and CaNa2EDTA.27-29 Moderate lead intoxication is defined as a BLL
of 45-69 mcg/dL without signs and symptoms related to lead toxicity.27-29
Chelation may be administered orally or parenterally. The use of chelators in
mild intoxication (10-44 mcg/dL) is not supported by the literature. All
chelators work by increasing the urinary excretion of lead.27-29
Intravenously administered
chelating agents are the treatment of choice for severe lead toxicity.
27-29 These agents include dimercaprol and CaNa2EDTA. Dimercaprol
(2,3-dimercapto-1-propanol, also known as British Anti-Lewisite (BAL),
was developed in 1946 and is the agent of choice in treating severe symptoms
of lead toxicity.29 Dimercaprol is administered as a deep
intramuscular injection. The usual dosage is 75 mg/m2 every four
hours for five days.28 It is contraindicated in patients with
hepatic insufficiency and in patients with peanut allergies.28,29
Dimercaprol should be used with caution in children with renal impairment,
hypertension, or G6PD deficiency.28,29 Adverse effects include
nausea, vomiting, headache, tachycardia, and leukopenia. Concomitant iron
therapy should be discontinued during chelation therapy due to increased
nausea and vomiting.29
CaNa2EDTA (calcium disodium
ethylenediamine tetra-acetate) was found to be useful in lead poisoning in the
1950s.29 CaNa2EDTA is considered second line after dimercaprol
because it may lead to increased lead concentration in the central nervous
system and, as a consequence, elevated intracranial pressure.27-29
CaNaEDTA may be administered intravenously or intramuscularly. Intravenous
administration allows for continuous chelation and is less painful than
intramuscular administration.28,29 The usual dosage is 1,000-1,500
mg/m2/day as a continuous infusion for five days.28
Adverse effects include local injection site reactions, fever, hypercalcemia,
renal insufficiency, and excretion of other essential minerals.28,29
CaNaEDTA was used to monitor response to chelator therapy as the EDTA
mobilization test. Costly and difficult to administer, this test is no longer
recommended.30
Several medications have the
active ingredient EDTA that leads to the potential for medication errors. One
such agent, Na2EDTA, edetate disodium (Endrate), should never be used for
treating lead poisoning. This chelating agent is used to treat hypercalcemia.
Its use in lead poisoning may lead to life-threatening hypocalcemia.31
DMSA, or succimer
(meso-2,3-dimercaptosuccinic acid), is a water-soluble analog of dimercaprol
that is administered orally and is associated with fewer adverse effects than
parenteral chelators.27-29 Adverse effects include rash,
neutropenia, elevated transaminases, and gastrointestinal upset.28
DMSA has a sulfur odor that may be masked by opening the capsules and
sprinkling the beads onto food or dissolving into juice.28,29 The
recommended dosage is 350 mg/m2 administered three times daily for
five days, then twice daily for 14 days.28
D-penicillamine, originally
developed to reduce copper concentrations in patients with Wilson's disease,
is used as an oral chelator in children with low-level toxicity. However, its
use has not been FDA approved.29,32 Adverse effects include nausea
and vomiting, transient neutropenia and thrombocytopenia, rash, abdominal
pain, and abnormal liver function.29,32 If angioedema, urticaria,
or a maculopapular rash occur, discontinuation of therapy may be necessary.
29
Chelators remove lead from
blood and tissue (including the brain).28,29 Chelation therapy may
reverse acute encephalopathy and alleviate vomiting, abdominal pain, anemia,
and renal insufficiency caused by lead intoxication. However, chelation
therapy does not affect the neurologic sequelae of chronic lead toxicity.
33-35 BAL or CaNa2EDTA as single agents in the treatment of acute severe
lead encephalopathy have been shown to reduce mortality from 66% to 30%.
34,35 In combination, mortality was further reduced to 1% to 2%.36
In moderate lead toxicity, a trial comparing CaNaEDTA and succimer
demonstrated that succimer was more effective in reducing mean BLL and was
well tolerated.37
There are no studies
evaluating the efficacy of chelators in adults with lead toxicity.12
The key to treatment is removal from exposure. Chelation may be considered on
a case-by-case basis in patients with a BLL greater than 80 mcg/dL, a BLL
between 60 and 80 mcg/dL with symptoms, or a BLL between 40 and 60 mcg/dL if
symptoms continue after removal from the source of lead exposure.38
The Role of the Pharmacist
The best treatment
against lead toxicity is prevention. Pharmacists should be aware of potential
lead sources in the community. If there is concern about possible lead
toxicity, the pharmacist should contact the local public health department and
poison control center. The importance of early identification of lead
toxicity sources cannot be overemphasized.
References
1. CDC's Third
National Report on Human Exposure to Environmental Chemicals: Spotlight on
Lead. NCEH Pub 05-0664. July 2005.
2. Roscoe RJ, Ball W,
et al. Adult Blood Lead Epidemiology and Surveillance---United States,
1998-2001. MMWR Surveillance Summary. 2002;51:1.
3. Adult blood lead
epidemiology and surveillance--United States 2002. MMWR Morbidity
Mortality Weekly Report 2004;53:578.
4. Shevell M, Ashwal S
et al. Practice parameter: evaluation of the child with global developmental
delay: report of the Quality Standards Subcommittee of the American Academy of
Neurology and the Practice Committee of the Child Neurology Society.
Neurology. 2003;60:367-380.
5. Bellinger D, Sloman
J, Leviton A, et al. Low-level lead exposure and children's cognitive function
in the preschool years. Pediatrics. 1991;87:219-227.
6. Gomaa A, Hu H,
Bellinger D, et al. Maternal bone lead as an independent risk factor for fetal
neurotoxicity: A prospective study. Pediatrics. 2002;110:110-119.
7. Adgate JL, Weisel C,
Wang Y, et al. Lead in house dust: Relationships between exposure metrics.
Environmental Research. 1995;70:134-147.
8. Lanphear BP, Burgoon
DA, Rust SW, et al. Environmental exposures to lead and urban children's blood
lead levels. Environmental Research. 1998;76:120-130.
9. Hurwitz RL, Lee DA.
Childhood lead poisoning: exposure and prevention. In: Rose BD, ed.
UpToDate.. Waltham, MA; 2007.
10. Silbergeld EK.
Implications of new data on lead toxicity for managing and preventing
exposure. Environmental Health Perspectives. 1990;89:49-54.
11. Shih RA, Hu H,
Weisskopf MG, et al. Cumulative lead dose and cognitive function in adults: a
review of studies that measured both blood lead and bone lead.
Environmental Health Perspectives. 2007;115:483-492.
12. Goldman RH, Hu H.
Adult lead poisoning. In: UpToDate, Rose, BD, ed. Waltham, MA; 2007.
13. Rischitelli G,
Nygren P, Bougatsos C, et al. Screening for elevated lead levels in children
and pregnancy: an updated summary of evidence for the US Preventive Services
Task Force. Pediatrics. 2006;118:e1867-1895.
14. Kuruvilla A, Pillay
VV, Adhikari P, et al. Clinical manifestations of lead workers in Mangalore,
India. Toxicology and Industrial Health. 2006;22:405-413.
15. Pearce JM. Burton's
line in lead poisoning. European Neurology. 2007;57:118-119.
16. Hurwitz RL, Lee DA.
Childhood lead poisoning: clinical manifestations and exposure. In: Rose BD,
ed.UpToDate. Waltham, MA; 2007.
17. Shiri R, Ansari M,
Ranta M, et al. Lead poisoning and recurrent abdominal pain. Industrial
Health. 2007;45:494-496.
18. Patrick L. Lead
toxicity, a review of the literature. Part I: exposure, evaluation, and
treatment. Alternative Medicine Review. 2006;11:2-22.
19. Lanphear BP,
Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead
concentrations <10 mcg/dL in US children and adolescents. Public Health
Reports. 2000;115:521-529.
20. Schwartz BS,
Byung-Kook L, Bandeen-Roche K, et al. Occupational lead exposure and
longitudinal decline in neurobehavioral test scores. Epidemiology.
2000;16:106-113.
21. Shih RA, Glass TA,
Bandeen-Roche K, et al. Environmental lead exposure and cognitive function in
community-dwelling older adults. Neurology. 2006;67:1556-1562.
22. Schaumberg DA,
Mendes F, Balaram M, et al. Accumulated lead exposure and risk of age-related
cataract in men. JAMA. 2004;292:2750-2754.
23. Hu H, Watanabe H,
Payton M, et al. The relationship between bone lead and hemoglobin. JAMA.
1994;272:1512-1517.
24. Martin D, Glass TA,
Bandeen-Roche K, et al. Association of blood lead and tibia lead with blood
pressure and hypertension in a community sample of older adults. American
Journal of Epidemiology. 2006;163:467-478.
25. Cheng Y, Schwartz
J, Sparrow D, et al. Bone lead and blood lead levels in relation to baseline
blood pressure and prospective development of hypertension. American
Journal of Epidemiology. 2001;153:164-171.
26. Muntner P, Menke A,
Batuman V, et al. Association of tibia lead and blood lead with end-stage
renal disease: a pilot study of African-Americans. Environmental Research.
2007;104:396-401.
27. American Academy of
Pediatrics Committee on Environmental Health. Lead exposure in children:
prevention, detection, and management. Pediatrics. 2005;116:1036-1046.
28. Garcia RC,
Snodgrass WR. Lead toxicity and chelation therapy. Am J Health-Syst Pharm
. 2007;64:45-52.
29. Hurwitz RL, Lee DA.
Childhood lead poisoning: Treatment. In: Rose BD, ed. UpToDate.
Waltham, MA; 2007.
30. Treatment
guidelines for lead exposure in children. American Academy of Pediatrics
committee on Drugs. Pediatrics. 1995;96(pt 1):155-160.
31. Agency for Toxic
Substances and Disease Registry. Case studies in environmental medicine
(CSEM). Lead toxicity. August 2007. Available at: www.atsdr.cdc.gov/csem.
Accessed September 21, 2007.
32. Liebelt EL, Shannon
MW. Oral chelators for childhood lead poisoning. Pediatr Ann.
1994;23:616-619,623-626.
33. Dietrich KN, Ware
JH, Salganik M, et al. Effect of chelation therapy on the neuropsychological
and behavioral development of lead-exposed children after school entry.
Pediatrics. 2004;114:19-26.
34. Rogan WJ, Dietrich
KN, Ware JH, et al. The effect of chelation therapy with succimer on
neuropsychological development in children exposed to lead. N Engl J Med
. 2001;344:1421-1426.
35. Perlstein MA,
Attala R. Neurologic sequelae of plumbism in children. Clin Pediatr.
1966;5:292.
36. Chisolm JJ. The use
of chelating agents in the treatment of acute and chronic lead intoxication in
childhood. J Pediatr.1968;73:1-38.
37. Graziano JH,
Lolacono NJ, Moulton T, et al. Controlled study of meso-2,3-dimercaptosuccinic
acid for the management of childhood lead intoxications. J Pediatr.
1992;120:133-139.
38. Kosnett MJ, Wedeen
RP, Rothenberg SJ, et al. Recommendations for the medical management of adult
lead exposure. Environ Health Perspect. 2007;115:463-471.
To comment on this article, contact editor@uspharmacist.com.





