US Pharm. 2015;40(2)(Specialty&Oncology suppl):6-8.
ABSTRACT: Lung cancer is one of the leading causes of death in the United States. The two major types of lung cancer are small cell lung cancer and non–small cell lung cancer (NSCLC); NSCLC is more common. Screening and prevention rates for lung cancer are poor compared with those for other types of cancer, but have improved in the past couple of years because of recent updates to lung cancer screening guidelines. Treatment varies depending upon the disease stage. The emergence of newer oral agents such as afatinib, crizotinib, nivolumab, and ceritinib has contributed immensely to lung cancer treatment. It is important for pharmacists to stay up-to-date on treatment agents in order to promote patients’ well-being.
Lung cancer is one of the leading causes of death in the United States. This review article will provide a general overview of lung cancer and focus on treatment options for lung cancer, with emphasis on screening guideline updates and oral chemotherapy agents.1
Background
Lung cancer is the second most common malignancy in the U.S. and the most common cause of cancer-related deaths. Only 15% of patients with lung cancer survive 5 years after diagnosis. Most cases of lung cancer are attributable to smoking tobacco. There are two types of lung cancer: small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC). SCLC and NSCLC differ in many ways. SCLC, which is the more aggressive form, occurs most often in smokers. Classifications of NSCLC are adenocarcinoma, squamous cell carcinoma, and large-cell lung cancer. NSCLC grows more slowly than SCLC, is more sensitive to radiation, and accounts for about 80% of all lung cancers. The oncogenes involved in NSCLC are c-Myc, c-Met, Bcl-2, p53, Rb, and other DNA chromosomal changes. Patients with NSCLC have a better prognosis compared with SCLC patients, since NSCLC is less aggressive. Patients with SCLC are more likely to present with metastatic disease in the brain, which is a common site of metastases (60%-70%).1,2
Risk Factors and Screening
Risk factors for developing lung cancer include smoking (>90% of cases), with the risk escalating with increased amount and/or duration of smoking; radon exposure; asbestos; diet (high in beta-carotene); concurrent lung disease; and genetic predisposition. Prior to the National Lung Screening Trial (NLST), the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines did not recommend routine screening for lung cancer; however, the NCCN now recommends screening in patients who are at high risk for developing lung cancer. This includes patients aged 55 to 74 years with a ≥30 pack-year history of smoking tobacco who currently smoke (or former smokers who quit within the past 15 years) and patients aged ≥50 years with a ≥20 pack-year history of smoking tobacco and one additional risk factor. These patients should be evaluated once per year via low-dose CT. There are no recommended preventive treatments for lung cancer; however, avoiding smoking dramatically reduces the risk of disease development.2
The decision to update the screening guidelines stemmed from results of the NLST, which was performed over 8 years by the National Cancer Institute and the American College of Radiology Imaging Network. Prior to the trial, it was unclear whether chest x-ray or low-dose CT was more effective for screening patients at high risk for developing lung cancer. A total of 53,454 participants at screening centers across the U.S. were randomized to receive low-dose CT or chest x-ray, and median follow-up was 6.5 years. The primary outcome measure was lung cancer deaths. Results were consistent with the literature, further validating that low-dose CT results in a reduction in mortality if the staff is experienced in performing chest CT.3
Signs and symptoms of both SCLC and NSCLC include cough, weight loss, dyspnea, chest pain, bronchitis, hemoptysis, hoarseness, bone pain, wheezing, and pneumonia. Diagnosis is made by obtaining a thorough history and physical examination, MRI, bone scans, and tissue sampling, which allows for staging. SCLC is classified as limited or extensive. Limited-stage disease is defined as the involvement of one hemithorax, the mediastinum, or regional lymph nodes that can be contained in a tolerable radiation port, whereas extensive-stage disease means that the disease has spread beyond the limited area. NSCLC is staged according to the traditional TNM (primary tumor, regional lymph nodes, and distant metastasis) system consisting of stages I, II, III, and IV.1,2
Treatment
The treatment of SCLC is based on stage, with the goal being cure; however, only about 40% of patients are still living 2 years after treatment. Surgery plays a limited role in SCLC treatment because this cancer grows rapidly—causing most patients to present with metastatic disease—and can recur in just a few months. Limited-stage disease is treated with chemotherapy, such as cisplatin and etoposide (considered first-line; carboplatin may be substituted for cisplatin, if needed), and concurrent radiation. All SCLC patients with limited-stage disease should receive prophylactic cranial irradiation (PCI), owing to the high possibility of brain metastases. Extensive-stage SCLC has a very low survival rate and is rarely curable; expected survival without treatment is about 5 weeks. It is treated with cisplatin or carboplatin plus etoposide or irinotecan. ICE-V (ifosfamide, carboplatin, etoposide, and vincristine) should be reserved as a last-line treatment option.2 The use of PCI in extensive-stage SCLC has been controversial; historically PCI was not recommended, but after a study demonstrated not only a decrease in risk of brain metastases (from 40.4% to 14.6%), but also prolonged survival, the guidelines were revised to include a recommendation for its use in extensive-stage SCLC.4
For NSCLC stages I, II, and III, surgery is the treatment of choice. Life expectancy in untreated NSCLC is about a year. External beam radiation has been used in patients who may be unable to tolerate surgery. Cisplatin-based regimens are the gold standard and have shown the most benefit. Radiation is given if there are positive margins after surgery, and it is used with chemotherapy if it meets criteria or the tumor is inoperable. Cisplatin and vinorelbine have exceptional results when given concurrently with radiation. Radiation is not always recommended; it depends on the stage subtype of NSCLC. Carboplatin and paclitaxel may also be used in the treatment of NSCLC. For stage IV metastatic disease, newer agents, such as erlotinib, crizotinib, and afatinib, are used. Cisplatin, paclitaxel, and bevacizumab are a second-line option for metastatic disease. Other agents that that may be used in conjunction with cisplatin are docetaxel, irinotecan, and nanoparticle albumin-bound paclitaxel.2
New Treatment Developments
Owing to the emerging increase in the occurrence of lung cancer, many trials are being conducted to develop more targeted therapies. Epidermal growth factor receptor (EGFR) mutations are found in about 30% of patients with NSCLC (typically more often in East Asians), which was the basis for the use of tyrosine kinase inhibitors (TKIs). EGFR-TKIs have a high objective response rate and longer progression-free survival (PFS) rates compared with chemotherapy alone, but patients tend to develop resistance, resulting in disease progression. Adverse events (AEs) most commonly seen with EGFR-TKIs are diarrhea, rash, and acne.5,6
Afatinib, an EGFR inhibitor, is effective against a gatekeeper mutation known as T790M. Afatinib is approved at a dosage of 40 mg once daily until disease progression in metastatic NSCLC with exon 19 deletions or exon 21 substitution mutations. The LUX-Lung 4 trial investigated afatinib therapy in patients aged ≥20 years who had confirmed stage III or IV adenocarcinoma and previously underwent ≥12 weeks of EGFR-TKI treatment. Afatinib showed significant efficacy after failure of erlotinib or gefitinib and one or two lines of chemotherapy. The results of LUX-Lung 4 led to other trials.5,6
In addition to EGFR-TKIs, newer drugs called programmed death receptor-1 (PD-1) antibodies are currently in clinical trials. PD-1 is a protein located on T cells whose normal pathway is disrupted when a tumor is present, causing immune-system suppression. One PD-1 agent, nivolumab, is being studied in chemotherapy-naïve patients as first-line treatment for advanced NSCLC; interim results indicate increases in PFS and overall survival, but more studies need to be conducted.7
Several trials are examining nivolumab administered with ipilimumab, erlotinib, and chemotherapy. Nivolumab dosages being studied are 0.1 mg/kg to 10 mg/kg every 2 weeks for 12- to 18-week cycles. Responses seem encouraging, but these trials are not yet completed, and additional trials must be conducted.7 Nivolumab is just one of many PD-1 antibody immunotherapies under investigation.
AEs of immunotherapies may require close attention, especially in oncology clinics. The most common AEs include pruritus, rash, diarrhea, hepatitis, and fever (TABLE 1). Despite the close monitoring necessary in patients receiving these agents, immunotherapies enable a wider selection of patients since they do not target a specific gene or mutation, which can be a huge advantage for those with NSCLC. Immunotherapies have extensive antitumor effects and may provide a novel treatment approach to lung cancer in the future.8
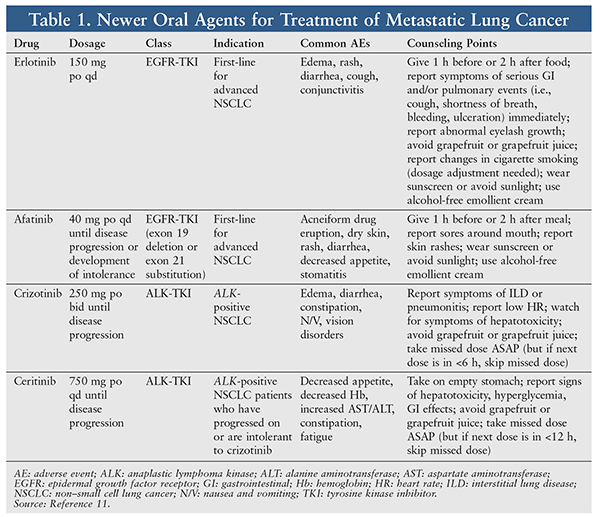
Another molecular gene subtype shown to have activity in lung cancer is the anaplastic lymphoma kinase (ALK) gene. This tyrosine kinase target is found in about 5% of cases of NSCLC. Crizotinib is an oral TKI that targets ALK-positive NSCLC. A study compared oral crizotinib 250 mg twice daily with a 3-week IV chemotherapy cycle of pemetrexed or docetaxel. Crizotinib showed a median PFS of 7.7 months versus 3 months in the chemotherapy group. For PFS, crizotinib was superior to standard chemotherapy in advanced ALK-positive NSCLC.9
These aforementioned landmark trials led to the addition of afatinib and crizotinib as first-line treatment for metastatic lung cancer in the NCCN guidelines.2
In April 2014, ceritinib—used in ALK-positive NSCLC patients—received approval for use in patients who progress on or are intolerant to crizotinib. Ceritinib inhibits autophosphorylation of ALK and impedes proliferation of ALK in cancer cells. Ceritinib was approved following a multicenter, open-label trial of 163 patients with ALK-positive metastatic NSCLC who were intolerant to or had progressed on crizotinib. The primary endpoint was objective response rate, and ceritinib showed promising results. Ceritinib has the added benefit of central nervous system penetration and response, which is important with lung cancer, since the brain is one of the most common sites of metastasis.10 However, the current wholesale cost of $13,500 per month may be a limiting factor.
Refer to TABLE 1 for a list of oral chemotherapy agents used in lung cancer. The trial drugs under development are targeted to specific genes, such as KRAS and EGFR. It is hoped that this development will decrease the AEs associated with chemotherapy and help control the disease more effectively.
Conclusion
Lung cancer is a leading cause of cancer-related death.3 The high incidence of mortality makes it vital to devote time, funds, and research to further developing treatment options for the different types of lung cancer. Different molecular origins of lung cancer result from environmental and genetic changes beyond human control. Early detection is vital, and the updates made to lung cancer screening guidelines validate its importance. Many patients fail treatment with chemotherapy agents, and a need for newer medications is vital.1 The emergence of newer oral agents, such as afatinib, crizotinib, nivolumab, and ceritinib, has contributed immensely to lung cancer treatment. To enhance patient well-being, it is essential for pharmacists to keep abreast of treatment agents.
ACKNOWLEDGMENT: The author thanks Nathan Greenfield, PharmD Candidate 2015, for his assistance on this article.
REFERENCES
1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-1380.
2. National Comprehensive Cancer Network. Lung cancer screening. NCCN Clinical Practice Guidelines in Oncology. Version 2.2014. www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed June 1, 2014.
3. National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991.
4. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-672.
5. Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012;13:539-548.
6. Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013;31:3335-3341.
7. Helwick C. Strong showing for anti–PD-1 agents in non–small cell lung cancer. ASCO Post. www.ascopost.com/issues/july-10,-2014/strong-showing-for-anti–pd-1-agents-in-non–small-cell-lung-cancer.aspx. Accessed July 16, 2014.
8. Davies M. New modalities of cancer treatment for NSCLC: focus on immunotherapy. Cancer Manag Res. 2014;6:63-75.
9. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385-2394.
10. Stenger M. Ceritinib in ALK-positive metastatic NSCLC patients with progression on or intolerance to crizotinib. ASCO Post. www.ascopost.com/issues/june-10,-2014/ceritinib-in-alk-positive-metastatic-nsclc-patients-with-progression-on-or-intolerance-to-crizotinib.aspx. Accessed July 17, 2014.
11. Micromedex Healthcare Series. DRUGDEX System. Greenwood Village, CO: Truven Health Analytics; 2013. www.thomsonhc.com. Accessed July 16, 2014.
To comment on this article, contact rdavidson@uspharmacist.com.






