US Pharm. 2019;44(11)(Specialty&Oncology suppl):11-15.
ABSTRACT: The immune system plays a key role in the regulation of tumor cells. The development of immune checkpoint inhibitors and the use of these agents over the past decade has increased the number of treatment options for patients with cancer. Immune checkpoint inhibitors present a novel set of immune-related adverse events in which any organ system may be affected, in some cases seriously. Dermatologic, gastrointestinal-tract, endocrine, and rheumatologic adverse events are the most common types. The treatment of immune-related adverse events depends on the organ system affected and the grade of severity. In the majority of cases, temporary discontinuation of immune checkpoint inhibitor therapy and administration of corticosteroids will mitigate the adverse events.
The immune system plays a primary role in the regulation of cells, including tumor cells, via various checkpoints. Malignant cells are recognized by the immune system as nonself antigens (i.e., foreign), and T cells bind to and interact with tumor cells to cause cell apoptosis. However, certain cancer cells have learned to evade detection by the immune system. One of the ways these cells avoid recognition involves their interaction with immune-system checkpoints.1 The two checkpoints that have generated the most interest over the past decade are the cytotoxic T lymphocyte–associated antigen-4 (CTLA-4) and programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) pathways. The CTLA-4 protein inhibits T-cell proliferation and subsequently downregulates the immune response to tumor antigens. Anti–CTLA-4 antibodies bind to CTLA-4 to inhibit interaction with its ligand, thereby restoring T-cell function. Likewise, PD-1 interacts with its ligands (PD-L1 and PD-L2) to inhibit T-cell function. Cancer cells and macrophages may express PD-L1, which is upregulated by interferon gamma. However, PD-L2 is expressed by dendritic cells and macrophages and is upregulated by interleukin 4.2
The development of immune checkpoint inhibitors (ICIs) and the use of these agents over the past decade has increased the number of treatment options for patients with cancer. Currently, seven FDA-approved ICIs are indicated for treatment of predominantly solid tumors, and indications for these novel options for cancer treatment are rapidly expanding as clinical-study results are published. Ipilimumab is the only approved anti–CTLA-4 antibody.3 Nivolumab, pembrolizumab, and cemiplimab are monoclonal antibodies that prevent PD-1 on T cells from binding to its ligands.4-6 Atezolizumab, durvalumab, and avelumab deter PD-L1 on tumor cells from binding to its corresponding receptor.7-9
This class of immunotherapy agents comes with its own set of unique adverse events (AEs). Unlike the AEs commonly associated with cytotoxic chemotherapy (i.e., neutropenia, anemia, alopecia, stomatitis, and myalgia), the AEs of checkpoint ICIs are similar to those for autoimmune disease and may affect any organ system.10 The dermatologic, gastrointestinal (GI) tract, endocrine, and rheumatologic systems are those most commonly affected by ICIs. Other organ systems affected—albeit rarely—are the pulmonary, renal, hematologic, cardiovascular, and ocular systems.11 In general, ICIs are better tolerated than cytotoxic chemotherapy and have a lower incidence of AEs. However, combined checkpoint blockade results in a higher incidence of AEs compared with ICI monotherapy. Additionally, an increased incidence of AEs with ipilimumab is linearly related to an increase in dose.11 The AE profiles of CTLA-4 and PD-1/PD-L1 agents are similar, but their incidences differ. Because ICIs have been on the market for a relatively short period of time, long-term AEs are still emerging. To better assist clinicians in managing AEs, guidelines have been developed by the National Comprehensive Cancer Network, the American Society for Clinical Oncology, the European Society for Medical Oncology, and the Society for Immunotherapy of Cancer. However, the recommendations of these various guidelines differ based on expert consensus and a growing body of literature, which must be taken into account in the management of immune-related AEs in this patient population. See TABLE 1 for detailed information on the management of immune-related AEs.
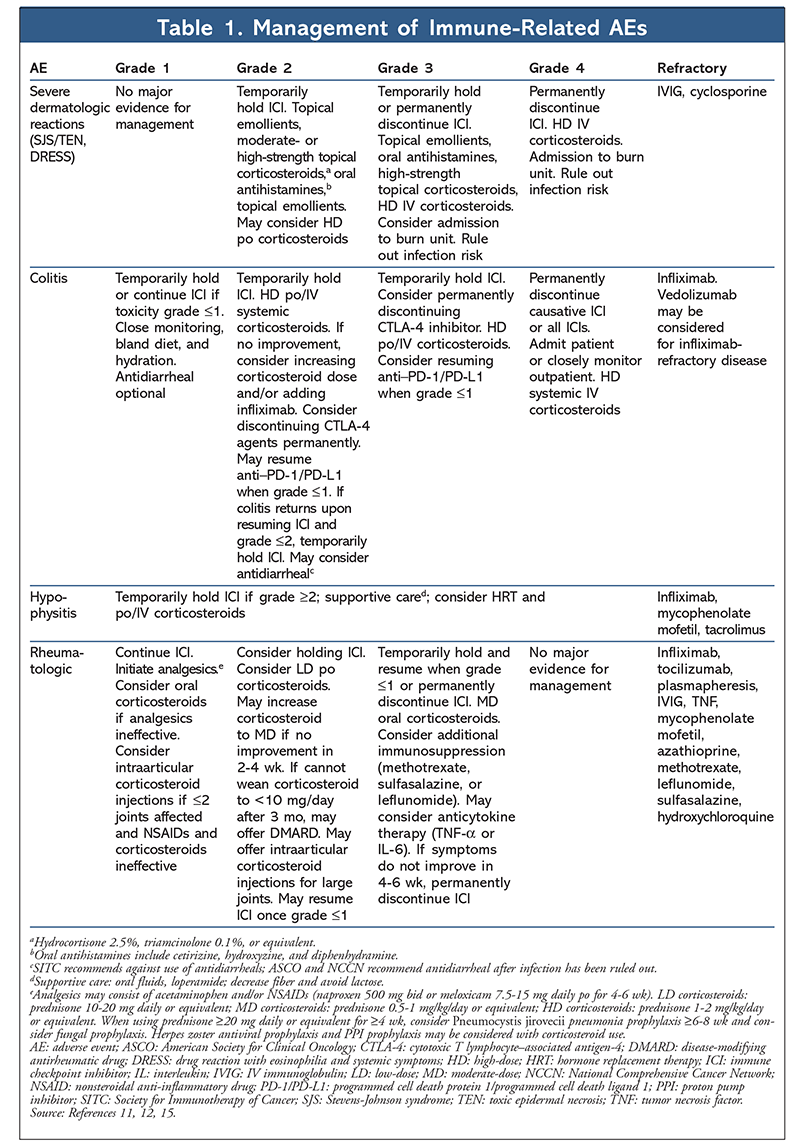
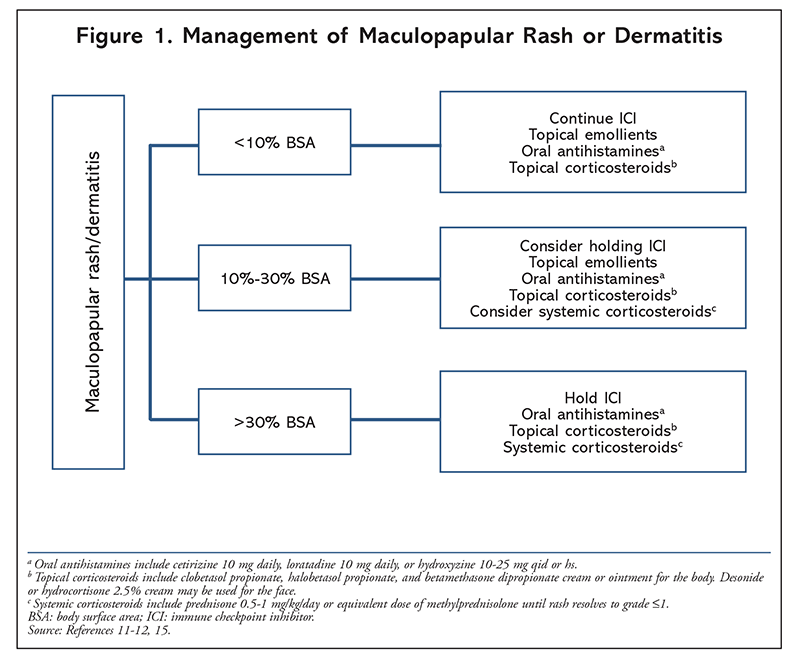
Dermatologic AEs
Dermatologic AEs occur in up to 50% of patients, and they are the most common immune-related AEs associated with ICIs.10,11 The presentation of cutaneous reactions to ICIs varies from inflammatory dermatitis to lichenoid and bullous dermatitis. Dermatologic reactions typically occur on the patient’s trunk and extremities. The time to onset of cutaneous AEs is typically within days to weeks of therapy initiation but on rare occasions may occur months after therapy initiation.12,13 General treatment for maculopapular rash includes topical emollients or topical corticosteroids for mild cases (FIGURE 1). In severe cases, oral corticosteroids such as prednisone may be used. If pruritus accompanies a rash, management may include antihistamines. If the cutaneous reaction is refractory to topical corticosteroids, a skin biopsy may be necessary to rule out other causes, and a dermatology consultation should be considered.11,13 Severe forms of cutaneous AEs, such as Stevens-Johnson syndrome, toxic epidermal necrosis, and drug reaction with eosinophilia and systemic symptoms, occur rarely. These severe reactions will require inpatient hospital admission and IV use of high-dose corticosteroids (TABLE 1). Once the symptoms are being controlled, a long oral-corticosteroid taper over 4 to 6 weeks will be necessary.14
GI-Tract AEs
Common ICI-related GI-system AEs include colitis and hepatitis. The clinical presentation of colitis varies from loose stools to abdominal pain caused by colonic inflammation; rarely, bloody diarrhea occurs. Colitis may present about 5 to 10 weeks after therapy initiation. Colitis is more common with ipilimumab or combination therapy with ipilimumab and nivolumab than with anti–PD-1/PD-L1 antibodies alone. Other causes of diarrhea, such as infection, Crohn’s disease, ulcerative colitis, and pseudomembranous colitis, should be ruled out.13 The grading of colitis severity depends on the number of stools above baseline per day. For grade 1, continuing ICI therapy is appropriate. However, depending on the clinical scenario, temporarily holding ICI therapy is an option. For grade 2 and higher, discontinuing ICI therapy and providing corticosteroids is appropriate. Guideline recommendations differ for permanent ICI discontinuation for grades 3 and higher. Additionally, in moderate-to-severe (grades 3-4) cases, corticosteroid therapy should be initiated while the GI workup is still in progress in order to prevent further treatment delays (TABLE 1).11,13,15
Colitis that is refractory to IV corticosteroids may be managed with infliximab. In most cases, a single dose of infliximab is effective and response should be seen in 1 to 3 days. If there is no improvement, a second infliximab dose may be administered after 2 weeks. In severe and refractory cases, maintenance infliximab therapy may be necessary.16 Vedolizumab should also be considered for corticosteroid-refractory colitis.13
Hepatitis is uncommon (<5%) with the use of PD-1/PD-L1 inhibitor monotherapy. However, the incidence is much greater (25%) when an anti–PD-1/PD-L1 drug is combined with ipilimumab.13 The patient may present with asymptomatic transaminitis and/or symptoms of fatigue, nausea, edema, and jaundice.17 The median time to onset is approximately 6 to 14 weeks.12 Patients with grade 1 toxicity may continue ICI treatment, but for grade 2 and higher, therapy should be discontinued and high-dose systemic corticosteroids should be initiated, such as IV methylprednisolone 1 to 2 mg/kg/day.14
In cases of corticosteroid-refractory hepatitis, mycophenolate mofetil may be used if there is no improvement within 5 to 7 days after corticosteroid initiation.10 Additionally, in cases of fulminant liver failure, antithymocyte globulin or IV immunoglobulin (IVIG) may be used.17 The use of infliximab for corticosteroid-refractory hepatitis is controversial because infliximab may have the potential to cause worsening hepatitis, according to its drug label.13
Endocrine AEs
Endocrine-related AEs include autoimmune dysfunction of the thyroid, pituitary, adrenal glands, and pancreas. The most common AE is thyroid dysfunction, with a hypothyroidism rate of 20%. The median time to onset of endocrinopathy ranges from 1 to 5 months.15 Typically, ICI-induced thyroiditis results in transient hyperthyroidism with subsequent irreversible hypothyroidism after approximately 1 month.12 Patients with symptomatic hyperthyroidism may benefit from therapy with a beta-blocker, such as atenolol 25 mg to 50 mg daily.11,12 Once a patient is considered to have hypothyroidism, thyroid replacement therapy (TRT) may be initiated with or without symptoms depending on the clinical scenario. Most likely, TRT will be necessary despite ICI cessation owing to the irreversible nature of hypothyroidism. Guideline recommendations vary for when to initiate TRT and when to temporarily hold ICI therapy. One similarity across all guidelines is that corticosteroid therapy is not recommended for hypothyroidism despite severity. Additionally, ICIs likely will not need to be permanently discontinued for hypothyroidism or hyperthyroidism. Because thyroid-related AEs are common, thyroid-function tests should be performed at baseline and every 4 to 6 weeks.15
Another AE of ICI therapy is hypophysitis (inflammation of the pituitary), which is more common with ipilimumab than with other ICIs.17 Patients typically present with headache and fatigue. Less common symptoms may include nausea, vertigo, visual changes, and weakness. The median time to onset is approximately 8 to 9 weeks after therapy initiation.12,14 The management of hypophysitis includes supportive care, high-dose corticosteroids, and ICI cessation (TABLE 1). Most cases of hypophysitis require long-term hormone replacement; few cases resolve spontaneously. For hypophysitis that is refractory to corticosteroid therapy after 72 hours, infliximab, mycophenolate mofetil, or tacrolimus may be initiated. Hypophysitis may result in both adrenal insufficiency and hypothyroidism, so treatment of adrenal insufficiency should be the priority in order to prevent an adrenal crisis.10
Adrenal insufficiency may also occur, although less commonly. Patients may present with hypotension, fatigue, and headaches. The primary management of adrenal insufficiency is cessation of ICI use and use of a stress-dose corticosteroid at the lowest dose to prevent symptoms from occurring. Supportive care with IV fluids may be required for hypotension.14,17 If an adrenal crisis occurs, IV fluids and high-dose corticosteroids should be initiated promptly, as this is a medical emergency.13
AEs affecting the pancreas include type 2 diabetes and, less commonly, type 1 diabetes. Patients will typically present with hyperglycemia, polyuria, and polydipsia. In cases of diabetic ketoacidosis, lethargy may also be present. If no known risk factors exist for diabetes and hyperglycemia is present, type 1 diabetes should be highly suspected. Oral agents for type 2 diabetes should be initiated for grade 1 toxicity; however, ICI use may be continued until the occurrence of at least grade 2 toxicity. As the toxicity grade increases, additional agents may be added for glucose control. For type 2 diabetes, insulin may be added to oral antihyperglycemic agents starting at grade 3 toxicity. If the type of diabetes is unknown or type 1 diabetes is suspected, insulin therapy should be first-line treatment. An endocrinology consultation is recommended for all patients with diabetes.11
Pulmonary AE
A rare but serious AE of ICIs is pneumonitis. The incidence is higher in non–small-cell lung cancer compared with melanoma.12 Clinical presentation may range from subtle symptoms such as dry cough or mild dyspnea to serious symptoms—i.e., shortness of breath or hypoxia. ICI use should be discontinued at any severity grade, and high-dose corticosteroids should be initiated in symptomatic patients.17 Patients may require empirical antibiotics and an infectious-disease consultation to rule out any infectious causes. If corticosteroid therapy is initiated, a gradual and cautious taper is necessary owing to the potential for exacerbation upon corticosteroid withdrawal.13,16 In patients who are refractory to corticosteroids after 48 hours, infliximab, mycophenolate mofetil, or IVIG may be initiated.12
Rheumatologic AEs
The incidence of rheumatologic AEs is approximately 2% to 12%, and these AEs are most commonly associated with PD-1/PD-L1 inhibitors.12,13 The most common presentations of rheumatologic and musculoskeletal AEs include inflammatory oligoarthritis and polyarthritis. Arthralgia may occur in up to 15% of patients undergoing ICI treatment.12 The median time to onset is approximately 5 months. For mild cases of arthralgia, mild analgesics may be used for symptom management. Severe rheumatologic AEs may be managed with moderate-dose corticosteroids.13,15 For arthritis that is refractory to corticosteroids after at least 2 weeks, infliximab, tocilizumab, and a variety of disease-modifying antirheumatic drugs may be considered for management (TABLE 1). Unfortunately, corticosteroid-refractory arthritis may persist for up to 2 years after ICI discontinuation.12 Although patients with autoimmune disease were excluded in clinical trials of ICIs, preexisting autoimmune disorders do not preclude ICI treatment.14
Conclusion
The mainstay of immune-related AE treatment is corticosteroid therapy. For refractory disease, agents such as infliximab, IVIG, cyclosporine, and anticytokine therapy, among others, have been successful in treating immune-related AEs. Management strategies for immune-related AEs from ICI therapy should become more sophisticated as long-term use of these agents increases. Additionally, the incidence and time to AE onset with combination CTLA-4 and PD-1/PD-L1 inhibition will be better understood as data continue to emerge. More research is needed to elucidate which populations may be at increased risk, such as patients with solid-organ transplants, allogeneic hematopoietic stem-cell transplants, or other significant preexisting conditions.
REFERENCES
1. Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3:250-261.
2. da Silva JL, Dos Santos ALS, Nunes NCC, et al. Cancer immunotherapy: the art of targeting the tumor immune microenvironment. Cancer Chemother Pharmacol. 2019;84(2):227-240.
3. Yervoy (ipilipumab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; September 2019.
4. Opdivo (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; September 2019.
5. Keytruda (pembrolizumab) package insert. Whitehouse Station, NJ: Merck & Co, Inc; September 2019.
6. Libtayo (cemiplimab) package insert. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; March 2019.
7. Tecentriq (atezolizumab) package insert. South San Francisco, CA: Genentech, Inc; May 2019.
8. Imfinzi (durvalumab) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; August 2019.
9. Bavencio (avelumab) package insert. Rockland, MA: EMD Serono, Inc; August 2019.
10. Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs. 2019;6(2): 154-160.
11. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768.
12. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95.
13. Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42(2):281-294.
14. Gerson JN, Ramamurthy C, Borghaei H. Managing adverse effects of immunotherapy. Clin Adv Hematol Oncol. 2018;16(5):364-374.
15. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Management of immunotherapy-related toxicities. Version 2.2019. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed August 1, 2019.
16. Martins F, Sykiotis GP, Maillard M, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20(1):e54-e64.
17. Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320(16):1702-1703.
To comment on this article, contact rdavidson@uspharmacist.com.





