US Pharm. 2013;38(11):49-50.
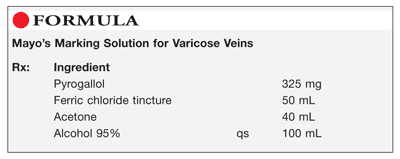
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Mix together the pyrogallol, ferric chloride tincture, and acetone. Add sufficient alcohol 95% to final volume and mix well. Package and label.
Use: This preparation has been used as a marking solution prior to varicose vein procedures.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period]. For external use only.
Stability: A beyond-use date of 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity (SG), active drug assay, color, clarity, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: Pyrogallol (pyrogallic acid, C6H6O3, MW 126.11) occurs as light, white, or nearly white, odorless leaflets or fine needles. It acquires a grayish tint upon exposure to air and light. When exposed to air, a solution of pyrogallol acquires a brown color and an acid reaction due to oxidation by air. This reaction proceeds more rapidly if the solution is alkaline. Pyrogallol has a melting point between 130°C and 133°C. It is used as a local antibacterial and local irritant, and it is an irritating skin antiseptic. Pyrogallol is soluble 1 g in 1.7 mL of water and 1.3 mL of alcohol. Aqueous solutions of pyrogallol oxidize and darken upon exposure to air, particularly if the solution is alkaline; the change is hastened by oxidizing agents. As with other phenolic compounds, pyrogallol provides coloration with iron salts. In neutral solution, pyrogallol forms precipitates with the salts of many metals.
Ferric chloride tincture (iron tincture) consists of ferric chloride solution (350 mL) made to volume with a sufficient quantity of alcohol to yield 1,000 mL. Ferric chloride tincture is a yellowish-orange liquid with a slightly ethereal odor, a highly astringent taste, and an acid reaction; it contains about 58% to 64% alcohol. Formerly, ferric chloride tincture was taken orally as a hematinic, but it is rarely used for this purpose today. It has been used locally as a styptic in surface bleeding.
Ferric chloride solution is an aqueous solution containing in each 100 mL not less than 37.2 g and not more than 42.7 g of iron(III) chloride, and not less than 3.85 g and not more than 6.6 g of hydrogen chloride. Ferric chloride solution is a yellowish-orange liquid with a faint odor of hydrochloric acid and an acid reaction. It is affected by light. Ferric chloride solution has been used as a hemostatic; its principal use, however, is as a pharmaceutical necessity to form (by dilution with alcohol) ferric chloride tincture.
Acetone (2-propanone, dimethyl ketone, C3H6O, MW 58.08) occurs as a transparent, colorless, mobile, volatile liquid with a characteristic odor. Formerly, it was obtained from the destructive distillation of wood; the distillate consisted principally of methanol, acetic acid, and acetone, which was neutralized with lime, and acetone was separated from the methanol by fractional distillation. Acetone is now largely obtained as a by-product of the butyl alcohol industry. A 50% aqueous solution is neutral to litmus. Acetone is miscible with water, alcohol, ether, and most volatile oils; it has an SG of not more than 0.789 and a boiling point of about 56°C. Acetone is used as a solvent or cosolvent in topical preparations up to a concentration of 13%, and as an aid in wet granulation. It is also used as an antiseptic and cleansing agent (in combination with alcohol).1,3
Alcohol 95% (ethyl alcohol, ethanol, grain alcohol, C2H5OH, MW 46.07) is a clear, colorless, mobile, volatile liquid with a slight, characteristic odor and a burning taste. It is used as an antimicrobial preservative (>10% concentration), a disinfectant (60-90% concentration), a solvent in injectable and oral liquids (variable concentration), and a solvent in topical products (60%-90% concentration). Alcohol USP refers to 95% ethanol; dehydrated alcohol refers to 99.5% alcohol. Alcohol’s SG is between 0.812 and 0.816, and its boiling point is 78.15°C. Alcohol is miscible with chloroform, glycerin, and water, and its solutions may be sterilized by autoclaving or filtration. It should be stored in a cool place. Alcohol is incompatible with oxidizing materials in acidic conditions. With alkalies, it may darken in color. When it is added to aqueous solutions of organic salts or acacia, they may precipitate. Alcohol is incompatible with aluminum containers, and it may interact with some drugs.4
REFERENCES
1. U.S. Pharmacopeia 36/National Formulary 31. Rockville, MD: U.S. Pharmacopeial Convention, Inc; 2013:335-398,1240.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. Kibbe AH. Acetone. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. London, England: Pharmaceutical Press; 2012:7-8.
4. Fenton ME. Alcohol. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. London, England: Pharmaceutical Press; 2012:19-22.
To comment on this article, contact rdavidson@uspharmacist.com.





