US Pharm. 2013;38(4):48-52.
ABSTRACT: Obesity is linked to health conditions including diabetes, hypertension, dyslipidemia, and cancer. Chronic drug therapy for obesity has been limited by cardiovascular (CV) and/or gastrointestinal adverse events (AEs), but two recently approved drugs—lorcaserin (Belviq) and phentermine and topiramate extended-release (Qsymia)—may prove helpful for achieving weight loss in obese patients. Lorcaserin is the first approved serotonin (5-HT)2C receptor agonist. The phentermine and topiramate extended-release combination is thought to work by suppressing appetite, reducing caloric intake and energetic efficiency, and increasing energy expenditure. The drugs’ efficacy cannot be compared directly at present, and it remains to be seen whether these agents are associated with an increased risk of CV AEs.
Current National Health and Nutrition Examination Survey data on obesity prevalence indicate that, in the period from 2009 to 2010, more than one-third of adults and almost 17% of children and adolescents were obese.1 The Healthy People 2010 goals of 15% obesity among adults and 5% obesity among children were not met, and the threat of obesity-related health conditions continues to rise.1 Obesity-related health conditions include type 2 diabetes (T2DM), hypertension, dyslipidemia, coronary heart disease (CHD), stroke, gallbladder disease, osteoarthritis, sleep apnea and respiratory problems, and certain types of cancer (endometrial, breast, prostate, and colon).2
FACTORS INVOLVED IN OBESITY
The pathophysiology of obesity is complex and multifactorial, involving physiological, genetic, and behavioral components. A certain amount of stored fat is required for survival during times of malnutrition or starvation. However, as weight increases, excess adipocytes release surplus free fatty acids, leading to lipotoxicity, insulin-receptor dysfunction, and hyperglycemia. In particular, white adipose tissue releases prefatty acids and adipokines, which are lipotoxic and inflammatory. The diverse effects of inflammatory adipokines include hyperglycemia, endothelial dysfunction, atheroma formation, plaque, and thrombosis. These injurious inflammatory secretagogues are countered by anti-inflammatory hormones such as adiponectin, visfatin, and acylation-stimulating protein.3
The influence of another factor—the obesogenic environment—on weight has yet to be fully understood. The obesogenic environment is “the sum of influences that the surroundings, opportunities, or conditions of life have on promoting obesity in individuals or populations.”4 Multiple factors, including the built environment (physical design, land-use patterns, transportation systems) and the food and nutrition environments (food access, advertising, marketing), must be addressed in order to truly make progress in the areas of obesity prevention and intervention.4
OBESITY GUIDELINES
An initial recommended loss of 10% of body weight during the first 6 months of a weight-loss regimen reduces many obesity-related health risks.2 The current (1998) obesity guidelines from the National Institutes of Health (NIH) include an Expert Panel’s Treatment Algorithm, which gives a step-by-step approach to managing weight loss in obese patients. Major components of this strategy include dietary therapy, increased physical activity, and behavior therapy. After 6 months of attempted lifestyle changes, pharmacotherapy may be considered as an adjunct in patients with a BMI ≥30 kg/m2 and no concomitant risk factors. Pharmacotherapy also may be considered in patients with a BMI ≥27 kg/m2 and an obesity-related risk factor or disease such as hypertension, dyslipidemia, CHD, T2DM, or sleep apnea.2 An update of the NIH obesity guidelines is in development, but it is unclear whether this resource will provide guidance on the use of the newer agents discussed in this article.
PHARMACOTHERAPY FOR OBESITY
Prior to the recent approvals of lorcaserin (Belviq, Eisai Inc.) and phentermine and topiramate extended-release (Qsymia, Vivus Pharmaceuticals), the only pharmacotherapeutic agent for long-term use was orlistat (approved in 1999). Orlistat is available both OTC (Alli) and by prescription (Xenical), but hepatotoxicity, as well as undesirable gastrointestinal adverse events (AEs)—oily spotting, abdominal pain/discomfort, flatus with discharge—in patients not following a low-fat diet, may limit use.5 Sibutramine (Meridia) was approved in 1997, but later was removed from the U.S. market based on concerns about cardiovascular (CV) safety raised by the Sibutramine Cardiovascular Outcomes Trial, which demonstrated a 16% increase in the risk of serious CV AEs. These serious AEs included nonfatal heart attack, nonfatal stroke, need for resuscitation once the heart stopped, and death in a group of patients given sibutramine versus a placebo group.6 Furthermore, the removal of the weight-loss drugs fenfluramine and dexfenfluramine in 1997 based on evidence that they were linked to heart-valve damage serves as a reminder that weight-loss drugs may prove efficacious, but are by no means without risks.7
The FDA has stipulated criteria for the development of new weight-loss medications. Clinical trials of weight-loss drugs must meet the following 1-year benchmarks in order for the drug to be considered effective: 1) The difference in mean weight loss between the active-product and placebo groups is at least 5 percentage points, with a statistically significant difference; and 2) the proportion of subjects in the active-product group who lose 5% or more of baseline body weight is at least 35% and approximately double the proportion in the placebo group, with a statistically significant difference between groups.8
RECENTLY APPROVED TREATMENTS: BELVIQ AND QSYMIA
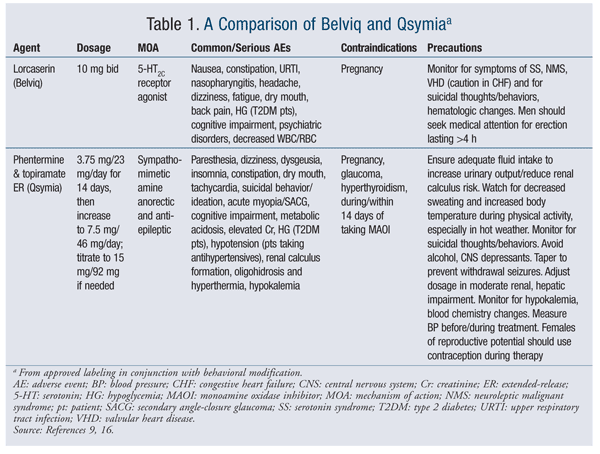
See TABLE 1 for a comparison of these agents.
Belviq
Lorcaserin, the first serotonin (5-HT)2C receptor agonist, was approved by the FDA on June 27, 2012, following an earlier rejection based on results of a preclinical study indicating that the drug may cause breast tumor in rats.7 Approved for chronic weight management, lorcaserin is thought to “decrease food consumption and promote satiety by selectively activating 5-HT2C receptors on anorexigenic pro-opiomelanocortin neurons located in the hypothalamus.”9 Human 5-HT2C receptors are distributed in areas of the brain that are associated with regulation of food intake, and 5-HT2C receptor knockout mice become hyperphagic, demonstrating partial leptin resistance, increased adiposity, insulin resistance, and glucose intolerance.10 The selectivity of lorcaserin for the 5-HT2C–receptor subtype is critical for obesity treatments, since activity at 5-HT2A and 5-HT2B may be associated with perceptual disturbances and valvular heart disease, respectively.11 Previously approved nonselective serotonergic agonists (fenfluramine and dexfenfluramine) validated serotonin receptors as pharmacologic targets for weight loss, but these agents were linked to serotonin-associated valvulopathy thought to result from agonism of 5-HT2B receptors expressed on cardiac valvular interstitial cells.12
Lorcaserin has the potential to bind at the 5-HT2A receptor, which has been associated with perceptual disturbances. In preclinical data, evidence of lorcaserin binding to this receptor is not strong, but a small proportion of patients who received supratherapeutic doses reported altered perception, abnormal dreams, sedation, or feelings of euphoria. Because of the novel mechanism of action and sporadic reports of AEs possibly associated with abuse potential, a study compared a single oral dose of lorcaserin (20, 40, and 60 mg) with zolpidem (15 and 30 mg), ketamine (100 mg), and placebo. This crossover study of recreational polydrug users found that subjective effects of lorcaserin 20 mg were similar to those of placebo, and supratherapeutic doses were associated with levels of dislike compared with placebo, zolpidem, and ketamine.11 Although this study demonstrated that lorcaserin does not appear to have behavior-modifying effects or significant abuse potential, two studies of abuse-related behavior in rats led the FDA to recommend that lorcaserin be classified as a Schedule IV controlled substance (CIV).13
Lorcaserin is indicated for chronic weight management in conjunction with a reduced-calorie diet and increased physical activity in patients with either an initial BMI ≥30 kg/m2 (obese) or an initial BMI ≥27 kg/m2 (overweight) and at least one weight-related comorbid condition such as hypertension, dyslipidemia, or T2DM. Lorcaserin is dosed at 10 mg twice daily, and common AEs include nausea, constipation, and upper respiratory infection.9
Qsymia
The phentermine and topiramate extended-release combination was approved by the FDA on July 17, 2012, almost 2 years following a previous rejection based on concerns over the drug’s potential to cause birth defects and the ways to mitigate these risks, as well as evidence that the heart-rate elevation associated with the combination might increase the risk of major CV AEs.14 The manufacturer’s proposed name for this product was Qnexa, which was disallowed by the FDA because of its strong similarity to other drug names.15
Phentermine and topiramate extended-release has the same indication as lorcaserin, i.e., for patients with either an initial BMI of ≥30 kg/m2 (obese) or an initial BMI of ≥27 kg/m2 (overweight) and at least one weight-related comorbid condition (e.g., hypertension, dyslipidemia, or T2DM), in conjunction with a reduced-calorie diet and increased physical activity.16 Phentermine as a single agent was previously approved as short-term (a few weeks) adjunctive therapy to a comprehensive weight-management program in obese patients with an initial BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with the presence of other risk factors. Phentermine is believed to suppress appetite by stimulating increased hypothalamic release of norepinephrine, but it has no detectable impact on serotonin. Animal studies suggest that the weight-loss effects associated with topiramate may result from reduced caloric intake, decreased energetic efficiency, and increased energy expenditure.17
Qsymia dosing, which requires upward titration, is initiated at 3.75 mg/23 mg daily for 14 days, then increased to 7.5 mg/46 mg daily. The patient should be evaluated after 12 weeks of taking the 7.5-mg/46-mg dosage. If the patient has not lost at least 3% of baseline body weight, Qsymia should be discontinued or the dosage should be escalated to 11.25 mg/69 mg for 14 days, followed by Qsymia 15 mg/92 mg. Weight loss should be reevaluated after an additional 12 weeks, and if the patient has not lost at least 5% of baseline body weight, Qsymia should be discontinued. Discontinuation should occur gradually (taking a dose every other day for 1 week prior to stopping treatment altogether) in order to avoid precipitating a seizure.16 The stepwise dosing regimen for Qsymia is significantly more complex than the regimen for Belviq, which involves simple twice-daily dosing.
Patient counseling should include the recommendation that Qsymia be dosed in the morning to avoid insomnia. This medication is scheduled as CIV based on the abuse potential of the phentermine component (recreational use or unhealthy weight loss). As with all sympathomimetic amines, it is necessary to monitor patients taking Qsymia for hypertension and tachycardia. In addition, since a fetus exposed to topiramate may experience teratogenic effects such as oral clefts, Qsymia is available only through a limited program under the Risk Evaluation and Mitigation Strategy. This program includes prescriber training, pharmacy certification, and a patient medication guide that details important safety information.16
Efficacy
Both Belviq and Qsymia were tested in placebo-controlled trials of different designs, but they have not been evaluated in a head-to-head clinical trial. Thus, it is not possible to directly compare the efficacy of these two medications.
A double-blind clinical trial that led to the approval of Belviq utilized primary outcomes of weight loss at 1 year and maintenance of weight loss at 2 years. Results showed that 45.7% of lorcaserin patients had lost at least 5% body weight at 1 year, compared with 20.3% of placebo patients. Of lorcaserin patients who lost more than 5% body weight during year 1, the loss was maintained during year 2 in 67.9%, versus in 50.3% of placebo patients. In addition, the rate of cardiac valvulopathy did not increase in the lorcaserin group.12
The clinical trial resulting in Qsymia approval involved men and women with class II (BMI 35-39.9 kg/m2) or class III obesity (BMI >40 kg/m2) who took phentermine and topiramate extended-release for 56 weeks and had primary outcomes of percentage of weight loss and proportion of patients achieving 5% weight loss. Patients taking the combination drug showed a dose-dependent mean change from baseline of –5.1 kg (3.75 mg/23 mg dosage) or –10.9 kg (15 mg/92 mg dosage) weight loss versus placebo patients (–1.6 kg weight loss). In addition, 45% of patients taking 3.75 mg/23 mg and 67% of patients taking 15 mg/92 mg lost at least 5% body weight versus 17% of placebo patients.17
CONCLUSION
The obesity pandemic is not merely a cosmetic issue, but a disease in itself that greatly increases the risk of other serious medical conditions, including diabetes, heart disease, and depression. The history of market withdrawals and CV AEs of obesity drugs may make practitioners hesitate to prescribe these drugs because of questions about the risk-to-benefit ratio. The FDA is requiring postmarketing studies of both new drugs, including a long-term CV-outcomes trial to assess the risk of major cardiac AEs such as heart attack and stroke.9,16 While it is exciting that the FDA has approved new obesity drugs for the first time in 13 years, the ultimate place of Belviq and Qsymia in weight-loss therapy has not been determined.
REFERENCES
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1-8.
2. The National Heart, Lung, and Blood Institute and the North American Association for the Study of Obesity. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 2000. NIH Publication No. 00-4084.
3. Redinger RN. The pathophysiology of obesity and its clinical manifestations. Gastroenterol Hepatol (N Y). 2007;3:856-863.
4. Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health. 2006;126:262-267.
5. Lexi-Comp Online [subscription database]. http://online.lexi.com. Accessed September 21, 2012.
6. U.S. Food and Drug Administration (FDA). Meridia (sibutramine):
market withdrawal due to risk of serious cardiovascular events.
www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm228830.htm.
Accessed September 21, 2012.
7. FDA. FDA approves Belviq to treat some overweight or obese
adults.
www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm309993.htm.
Accessed September 21, 2012.
8. Center for Drug Evaluation and Research. Guidance for Industry: Developing Products for Weight Management. Rockville, MD: FDA; 2007.
9. Belviq (lorcaserin) product information. Woodcliff Lake, NJ: Eisai, Inc; June 2012.
10. Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel
selective human 5-hydroxytryptamine 2C agonist: in vitro and in vivo
pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577-587.
11. Shram MJ, Schoedel KA, Bartlett C, et al. Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2c) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther. 2011;89:683-692.
12. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
13. Lorcaserin hydrochloride: briefing document for FDA Advisory
Committee meeting.
www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM303200.pdf.
Accessed September 21, 2012.
14. FDA. FDA approves weight-management drug Qsymia.
www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312468.htm.
Accessed September 18, 2012.
15. Miller R. FDA rejects Qnexa, asks for more info. www.theheart.org/article/1142017.do. Accessed September 18, 2012.
16. Qsymia (phentermine and topiramate extended-release) product information. Mountain View, CA: Vivus, Inc; July 2012.
17. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release
phentermine/topiramate in severely obese adults: a randomized controlled
trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
To comment on this article, contact rdavidson@uspharmacist.com.






