US Pharm. 2009;34(1)(Oncology suppl):10-14.
ABSTRACT: Oral mucositis (OM) is one of the most commonly occurring adverse effects in patients with head-and-neck cancer receiving chemotherapy (CT) and radiotherapy (RT). Compared with men, women have a significantly greater risk of OM; it usually is more severe and has a longer duration of action. Type of CT agent and RT also influence the chances of developing OM. Currently considered to be a subepithelial and epithelial injury, OM is believed to have five phases: initiation, signaling, amplification, ulceration, and healing. The proper use of assessment scales, nonpharmacologic methods such as good oral hygiene and cryotherapy, and pharmacologic treatment are important for the prevention or management of OM.
Oral mucositis (OM) is one of the most common adverse effects encountered during chemotherapy (CT) and radiotherapy (RT) in patients with head-and-neck cancer (HNC).1-4 Managing OM relies on the use of valid assessment scales, supportive care, symptom relief, and patient education. The condition can be debilitating, resulting in ulcers, severe pain, and dryness of the mouth that can lead to an inability to swallow. This can progress to malnutrition and the need for total parenteral nutrition (TPN), and as a result is one of the main reasons for interruption or early discontinuation of CT or RT.5,6 Since these effects negatively impact health-related quality of life, it is extremely important that OM be prevented whenever possible, or at least treated to reduce its severity and possible complications.7
Incidence
Current head-and-neck RT protocols have an OM incidence of 85% to 100%.7 The incidence of OM can approach 90% to 100% in patients receiving aggressive myeloablative CT. In patients with solid tumors who had CT-induced myelosuppression, OM occurred during 37% of 1,236 cycles of chemotherapy. The severity of OM depends on various factors, including the anticancer treatment protocol (TABLE 1), the patient’s age and diagnosis, the level of oral hygiene during therapy, and genetic factors.5,6,8

Risk Factors
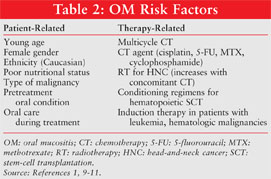
Patient-related factors such as age, gender, genetic factors (increased proinflammatory cytokine expression), preexisting poor oral hygiene, periodontal disease, nutritional status, RT- or drug-induced xerostomia, impaired salivary function, myelosuppression, tobacco use, and alcohol consumption have a major influence on the development, severity, and duration of OM (TABLE 2).1,9-11 Clinical data on how age influences OM have produced variable conclusions because few protocols are used across a large enough age range to allow for adequate comparisons. A significantly greater risk of OM has been found in women than in men (86% vs. 60%), and their OM generally is more severe and lasts longer.10 African-American patients treated with 5-fluorouracil (5-FU) for colon cancer have been found to experience less OM than Caucasian patients.11 Patients with poor nutritional status are at higher risk for developing OM.10 Treatment-related risk factors that increase the probability of developing OM are the type of CT agent (dose and schedule) and type of RT (location, dose, schedule, concomitant CT). There is a greater risk of OM with myelotoxic conditioning regimens required prior to hematopoietic stem-cell transplantation (SCT) and induction therapy in patients with leukemia or hematologic malignancies. Drugs affecting DNA synthesis (S phase–specific agents such as 5-FU, methotrexate [MTX], cyclophosphamide, and cytarabine) exhibit the most pronounced effects.9
Pathophysiology
Originally, OM was thought to occur as a result of direct and indirect toxic effects on epithelial cells.8 Today, OM is recognized as an epithelial and subepithelial injury and is believed to develop in a five-phase model7:
Phase 1—Initiation: Following the administration of CT or RT, DNA damage occurs in the basal epithelial cells. This causes breaks to occur in DNA strands within target cells in the mucosal epithelium and in the mucosa. This process generates reactive oxygen species (ROS), which can directly damage cells, and consequently leads to the development and progression of OM.
Phase 2—Signaling: CT, RT, and ROS cause DNA damage and subsequent cell death in the epithelium of the mucosa. They also activate various transcription factors, leading to increased production of inflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1 beta, and to apoptosis.
Phase 3—Amplification: The inflammatory cytokines (tumor necrosis factor–alpha, interleukin-1 beta) produced during phase 2 stimulate cells in the mucosa through positive-feedback loops that amplify the original signals triggered by the CT- and RT-induced damage. These cytokines cause further tissue damage, amplifying signaling cascades and the injury process.
Phase 4—Ulceration: The loss of mucosal integrity produces extremely painful lesions, resulting in the lack of oral alimentation. Breaks in the mucosal epithelium may provide portals of entry for bacteria, viruses, and fungi. Bacterial cell-wall products induce immune cells to produce cytokines, leading to further inflammation and apoptosis.
Phase 5—Healing: In the final stage, signals from the mucosa are thought to initiate healing of the epithelium. The proliferation and migration of epithelial cells restore the integrity of the mucosa until it appears normal.
OM Assessment Scales
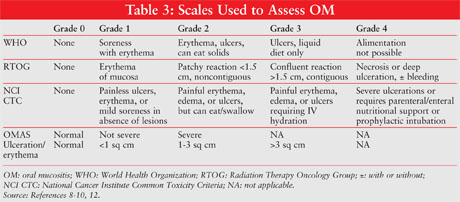
The most commonly used assessment scales for OM are summarized in TABLE 3. The World Health Organization (WHO) Oral Toxicity Scale measures anatomical, symptomatic, and functional components of OM.8 The severity of the condition is graded from 0 (no OM) to 4 (alimentation not possible; patient needs TPN). By contrast, the Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Scoring Criteria (ARMSC) for mucous membranes measure only the anatomical changes associated with OM.12 The National Cancer Institute (NCI) created the Common Toxicity Criteria (CTC) for the evaluation of CT-related effects, and the RTOG ARMSC constitute an evaluation of RT effects.9 The Oral Mucositis Assessment Scale provides an objective assessment of OM based on scoring of the presence and size of ulcerations.10
Nonpharmacologic Management
Routine Oral Care: Patients at risk for OM should be advised to brush with a soft-bristle toothbrush and gentle toothpaste two or three times a day. Use of an alcohol-free mouthwash is recommended, as is once-daily flossing. The patient should frequently assess the mouth for redness, swelling, sores, and areas of pain.
Cryotherapy: Cryotherapy is an inexpensive, readily available therapy that has been utilized for the prevention of OM in cancer patients. It is theorized to cause vasoconstriction, which decreases the amount of blood flow to the oral mucosa, thereby decreasing the amount of cytotoxic drug that reaches the oral mucosa. One unavoidable fault of studies assessing the efficacy of cryotherapy is the inability to blind patients and providers to the care the patients are receiving. Cryotherapy is unrivaled by any other management option in terms of availability and cost.
Cryotherapy was compared with rinsing with normal saline in 40 patients scheduled to undergo peripheral blood SCT who received melphalan two days prior to the procedure.11 The cryotherapy arm was instructed to place 1 oz. of ice chips in the mouth 30 minutes before the 30-minute melphalan infusion and let the ice melt completely, and to continue this process for six hours. Patients in the saline-rinse arm were instructed to swish 1 oz. of normal saline in the mouth every 30 minutes for six hours. The severity of OM was graded with the NCI CTC. Fourteen percent of patients in the cryotherapy group experienced Grade 3 OM compared with 74% of the saline group (P =.0005). The duration of OM also was less in the cryotherapy group than in the saline group (0.5 days vs. 4.6 days; P =.0001). Notably, noncompliance occurred with the prescribed length of cryotherapy treatment. Although length of stay (LOS) did not differ significantly between the two groups (cryotherapy, 9 days; saline, 14 days), there was a significant difference in days of IV narcotic use (cryotherapy, 0 days; saline, 5.5 days; P =.0003) and days on TPN (cryotherapy, 2 days; saline, 5.5 days; P =.04). Self-reports showed no difference in duration of mouth sores, but less mouth pain was reported in the cryotherapy group (P =.03).
Cryotherapy is not appropriate for all CT agents used in patients undergoing SCT. MTX is one such example. Gori et al randomized 130 patients undergoing SCT and receiving at least three doses of MTX to receive either cryotherapy or no intervention for one hour at the start of MTX administration.13 No difference in the duration or severity of OM was found between groups in this well-designed prospective trial.
Pharmacologic Management
Palifermin: Palifermin (Kepivance), which was FDA-approved in 2004 for the management of OM (TABLE 4), is a human recombinant keratinocyte growth factor that stimulates the development of cells on the surface of the gastrointestinal tract.14 In a double-blind, randomized, placebo-controlled trial conducted by Spielberger et al, patients received either palifermin 60 mcg/kg/day IV or placebo three days before and three days after autologous hematopoietic SCT.15 WHO Grade 3/4 OM was observed in 67 of the 106 patients (63%) in the palifermin group versus 104 of the 107 patients (98%) in the placebo group (P <.001). Among the patients who developed WHO Grade 3/4 OM, the median duration was 3.0 days in the palifermin group versus 9.0 days in the placebo group (P <.001). The palifermin group had a significantly lower rate (P <.001) and duration (P =.004) of WHO Grade 4 OM. Only one treatment-related adverse effect occurred in the palifermin group (rash) and in the placebo group (hypotension).
The Spielberger trial found that the reduced incidence of OM led to a decrease in opioid analgesic use and a decreased LOS. The economic ramifications of these findings were quantified in a study by Elting et al.16 Although cost information was not collected, costs were approximated from the National Inpatient Survey (NIS), a public claims database managed by the Agency for Healthcare Research and Quality. The 212 patients from the Spielberger trial were matched to 877 patients from the NIS. The estimated total cost per patient for a patient treated with palifermin was $73,938, versus $77,533 for patients treated with placebo. An increase in total cost was not associated with the use of palifermin despite the cost of the product.
Palifermin is also approved for use in patients with HNC. A randomized, placebo-controlled trial evaluated the safety and efficacy of palifermin in patients with HNC, with a follow-up observational study of survival.17 All patients received an RT and CT regimen that included cisplatin and 5-FU. Patients were randomized to receive palifermin 60 mcg/kg (67 patients) or placebo (32 patients) one week before undergoing chemoradiation therapy and once weekly for seven weeks after each radiation session plus two additional weeks (total of nine weeks). Duration of Grade 2 or greater OM—the primary endpoint—was not significantly shorter in the palifermin group at 6.5 weeks versus the placebo group at 8 weeks. Palifermin appeared to be more favorable for reducing the incidence and duration of OM in patients receiving hyperfractionated RT than in those receiving standard RT (P values not determined). No significant difference between groups was noted in the incidence of xerostomia and dysphagia. The trial concluded that palifermin was safe, as only two patients receiving palifermin were determined to have a serious treatment-related side effect, but higher palifermin doses with a more standardized assessment of OM were recommended.
The use of palifermin in patients undergoing treatment for colorectal cancer with 5-FU and leucovorin was evaluated in a randomized, placebo-controlled trial.18 Three days prior to each of the two five-day CT cycles, palifermin 40 mcg/kg or placebo was administered to 28 and 36 patients, respectively. Incidence, severity, and duration of OM; safety; and patient-reported outcomes were evaluated after adjustment for baseline Eastern Cooperative Oncology Group scores, prior CT, and carcinoembryonic antigen concentrations. In the first cycle, there was a 29% incidence of Grade 2 or greater OM in the palifermin group compared with 61% in the placebo group. The incidence of Grade 2 or greater OM was decreased in cycle 2, with an 11% incidence in the palifermin group versus 47% in the placebo group. Self-reported mouth and throat soreness scores were higher in placebo patients than in palifermin patients (P =.005). Oral adverse effects such as taste disorders were reported more commonly by the palifermin group than by the placebo group in both cycles, but the difference was not significant.
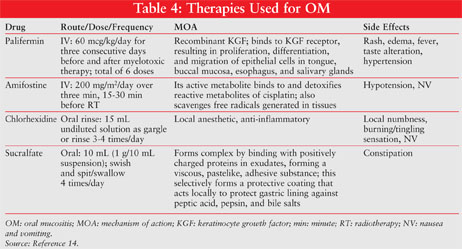
Amifostine: Amifostine (TABLE 4), an organic thiophosphate that is used as a normal-tissue protector during cytotoxic therapy, is recommended by current Multinational Association of Supportive Cancer Care (MASCC) guidelines for the prevention of radiation proctitis.14 Although it is not presently recommended for the treatment of OM, its effect on OM has been studied in patients undergoing SCT who received melphalan.19 In the 90 patients randomized to either amifostine or control, amifostine use was associated with a reduction in median grade of OM (WHO Grade 1 vs. 2; P =.01). Fewer patients (P =.02) experienced severe OM (WHO Grade 3/4) in the amifostine group (12%) compared with the control group (33%). Notably, 65% of patients in the amifostine arm experienced a toxicity related to the amifostine infusion, with one major toxicity (severe hypotension).
Chlorhexidine: Chlorhexidine (TABLE 4), an oral broad-spectrum antibiotic rinse, is not recommended by the National Comprehensive Cancer Network (NCCN) for prevention of OM in patients with solid tumors of the head and neck who are undergoing RT.14 Although colonization of microorganisms may be reduced with the use of chlorhexidine, a clinical benefit has not been demonstrated in large randomized, controlled trials.20 In addition, chlorhexidine mouthwash has been associated with an increase in oral mucosal inflammation and OM assessment scores, general mouth discomfort, taste alterations, and staining of teeth.21
A study published shortly after the January 2008 NCCN updated guidelines compared chlorhexidine versus cryotherapy in 206 patients with gastrointestinal malignancies who were scheduled to receive 5-FU.22 Patients were randomized to chlorhexidine 10 mL swirled around the mouth three times daily, cryotherapy for 45 minutes starting at the beginning of the infusion, or normal saline 10 mL three times daily (placebo). Incidence of Grade 3/4 OM in the chlorhexidine and cryotherapy groups was significantly less than in the placebo group (13%, 11%, and 33%, respectively). Duration of OM in the chlorhexidine and cryotherapy groups was also significantly less than in the placebo group (3 days, 1 day, and 5 days, respectively). There was no difference in incidence and duration of OM between the chlorhexidine and cryotherapy groups. Contrary to the NCCN guidelines, this study demonstrated that chlorhexidine was effective for the prevention of OM, but the authors suggested that the results need to be further evaluated.
Magic Mouthwash: Magic Mouthwash is thought to be effective for OM treatment owing to its pain-relieving properties and its coating of the mucosa. It can be composed of various ingredients, but usually is made of lidocaine, diphenhydramine, and an antacid containing aluminum/magnesium hydroxide in equal parts.23 A study that examinined the efficacy and safety of Magic Mouthwash (lidocaine, diphenhydramine, Maalox) versus a salt-and-soda rinse and chlorhexidine found no difference in efficacy between these agents.24
Antimicrobial Lozenges: Antimicrobial lozenges have been utilized based on the theory that they prevent bacterial complications of OM, but they are no longer recommended for the prevention of radiation-induced OM. A study comparing polymyxin, tobramycin, and amphotericin B versus placebo found no difference in the prevention of the development of severe OM in patients undergoing head and neck irradiation.25
Sucralfate: Sucralfate (TABLE 4) as a coating agent has been theorized to be useful in the management of OM, but without evidence to support its use, the NCCN and MSS MASCC do not recommend it for the treatment of RT-induced OM. A study comparing a sucralfate oral rinse with a salt-and-soda rinse in patients with HNC found no difference in number of days to OM onset, pain scale, or time to heal, and concluded that the salt-and-soda rinse was more cost-efficient and prudent.26
Review of Current Guidelines
In January 2008, the NCCN updated its guidelines for the prevention and treatment of OM.27 These guidelines were based on a literature review and the 2007 MASCC guidelines. For prevention, cryotherapy is recommended in patients receiving bolus-dosed 5-FU or edatrexate therapy or high-dose melphalan before hematopoietic SCT. Bland oral rinses should be used to provide mild symptomatic relief, moisturize tissues, and remove debris. Palifermin is recommended as preventive therapy in patients receiving conditioning regimens involving total-body irradiation before autologous SCT. Amifostine is recommended for prevention of xerostomia related to head and neck irradiation because it can reduce OM associated with high-dose melphalan. Topical oral antimicrobials (rinses or lozenges) should not be used as a preventive measure for OM; however, they can reduce microbial colonization (e.g., bacterial dental plaque) when routine oral hygiene is not possible. For treatment, bland rinses can be used for mild-to-moderate OM pain on an as-needed basis. To treat more severe OM pain, topical anesthetics can be used for breakthrough pain until analgesics can be administered and take effect. In myelosuppressive therapy, prophylactic antiviral and antifungal therapy may be considered to prevent infections. Alcohol-containing mouthwashes should be avoided.
REFERENCES
1. Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(suppl 9):1995-2025.
2. Epstein JB, Schubert MM. Oropharyngeal mucositis in cancer therapy. Review of pathogenesis, diagnosis, and management. Oncology. 2003;17:1767-1779.
3. Elting LS, Cooksley C, Chambers M, et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531-1539.
4. Sonis ST, Fey EG. Oral complications of cancer therapy. Oncology. 2002;16:680-686,691,692-695.
5. Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253-262.
6. Elting LS, Calais G, Selva N, et al. Patient-reported burden of mucosal injury (MUI): comparison of clinician-rated MUI and patient-reported outcomes [ASCO abstract 9117]. J Clin Oncol. 2007; 25(suppl 1).
7. Sonis S, Elting L, Keefe D. Burden of illness and economic impact of mucosal injury (MUI) in solid tumour—a multinational prospective observational study design [MASCC abstract ]16-101. Support Care Cancer. 2006;14:633.
8. World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization; 1979:15-22.
9. National Cancer Institute Cancer Therapy Evaluation Program. Common Toxicity Criteria Manual, Version 2.0. June 1, 1999. http://ctep.cancer.gov/forms/
10. Sonis ST, Eilers JP, Epstein JB, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85:2103-2113.
11. Lilleby K, Garcia P, Gooley T et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:1031-1035.
12. Olson K, Hanson J, Hamilton J, et al. Assessing the reliability and validity of the revised WCCNR stomatitis staging system for cancer therapy-induced stomatitis. Can Oncol Nurs J. 2004;14:168-174,176-182.
13. Gori E, Arpinati M, Bonifazi F, et al. Cryotherapy in the prevention of oral mucositis in patients receiving low-dose methotrexate following myeloablative allogeneic stem cell transplantation: a prospective randomized study of the Gruppo Italiano Trapianto di Midollo Osseo nurses group. Bone Marrow Transplant. 2007;39:347-352.
14. Lexi-Comp Online. Palifermin, amifostine, chlorhexidine, sucralfate. http://online.lexi.com/ 2004;14:168-174,176-182.
15. Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598.
16. Elting LS, Shih YC, Stiff PJ et al. Economic impact of palifermin on the costs of hospitalization for autologous hematopoietic stem-cell transplant: analysis of phase 3 trial results. Biol Blood Marrow Transplant. 2007;13:806-813.
17. Brizel DM, Murphy BA, Rosenthal DI, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489-2496.
18. Rosen LS, Abdi E, Davis ID, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol. 2006;24:5194-5200.
19. Spencer A, Horvath N, Gibson J, et al. Prospective randomised trial of amifostine cytoprotection in myeloma patients undergoing high-dose melphalan conditioned autologous stem cell transplantation. Bone Marrow Transplant. 2005;35:971-977.
20. Cheng KK, Chang AM, Yuen MP. Prevention of oral mucositis in paediatric patients treated with chemotherapy; a randomised crossover trial comparing two protocols of oral care.
Eur J Cancer. 2004;40:1208-1216.
21. Pitten FA, Kiefer T, Buth C, et al. Do cancer patients with chemotherapy-induced leukopenia benefit from an antiseptic chlorhexidine-based oral rinse? A double-blind, block-randomized, controlled study. J Hosp Infect. 2003;53:283-291.
22. Sorensen JB, Skovsgaard T, Bork E, et al. Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based chemotherapy-induced oral mucositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer. 2008;112:1600-1606.
23. Chan A, Ignoffo RJ. Survey of topical oral solutions for the treatment of chemo-induced oral mucositis. J Oncol Pharm Pract. 2005;11:139-143.
24. Dodd MJ, Dibble SL, Miaskowski C et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:39-47.
25. Stokman MA, Spijkervet FK, Burlage FR, et al. Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: a double-blind randomised clinical trial. Br J Cancer. 2003;88:1012-1016.
26. Dodd MJ, Miaskowski C, Greenspan D, et al. Radiation-induced mucositis: a randomized clinical trial of micronized sucralfate versus salt & soda mouthwashes. Cancer Invest. 2003;21:21-33.
27. Bensinger W, Schubert M, Ang KK, et al. NCCN Task Force Report. Prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(suppl 1):S1-S21.
To comment on this article, contact rdavidson@jobson.com.





