US Pharm. 2010;35(11):HS-22-HS-24.
To make healthy red blood cells, the human body needs to have enough iron. The most common cause of anemia in the United States is iron deficiency, which is resolved by eating iron in a healthy diet, taking iron pills, and, in severe cases, receiving iron injections. To determine if a patient is a candidate for iron injections, a physician will examine the patient to determine present symptoms, medical history, and any current treatments patients are receiving for iron
deficiency or anemia.
Iron in the red blood cells is necessary to move oxygen to the muscles and organs. Without an ample and steady supply of iron in the diet, however, iron deficiency and, in some cases, iron deficiency anemia, can develop. These conditions can make a person look pale and feel tired, weak, or dizzy. If these symptoms are related to anemia, eating a good diet including meat, leafy green vegetables, and iron-fortified grains may resolve the issue. In some cases, the body may not be absorbing iron properly or may need a lot more iron to make enough red blood cells. That means patient will need to receive additional iron, either as an iron pill taken orally or an iron injection. Patients who are pregnant most likely need some kind of iron supplementation.1
Blood Iron Tests
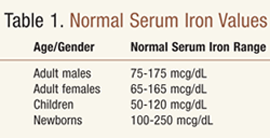
Serum Transferrin Test: This test measures blood iron level based on serum transferrin, which is a protein in the blood plasma. Normal serum iron values are shown in TABLE 1. Iron levels in serum are decreased in iron deficiency anemia, chronic blood loss, thyroid deficiency, chronically heavy menstrual periods, late pregnancy, and certain chronic diseases (e.g., arthritis). Certain medications can also cause decreased iron levels, such as ACTH, colchicine, deferoxamine, methicillin, and testosterone.2,3
Iron levels in serum are increased by multiple blood transfusions and intramuscular (IM) iron injections. Also, serum iron levels are increased in acute leukemia, thalassemia, hemochromatosis, severe hepatitis, lead poisoning, and kidney disease. Medications and substances that can cause increased iron levels include chloramphenicol, estrogen preparations, dietary iron supplements, alcoholic beverages, methyldopa, and birth control pills.2,3 Iron levels above 350-500 mcg/dL are considered toxic; levels over 1,000 mcg/dL indicate severe iron poisoning.
Ferritin Test: This test measures the level of a protein in the blood that stores iron for later use by the body. Dietary iron supplements can increase ferritin levels. In addition, some diseases that do not directly affect the body's iron storage can cause artificially high ferritin levels. These disorders include infections, late-stage cancers, lymphomas, and severe inflammations. Alcoholics often have high ferritin levels. Normal ferritin values are as follows: adult males, 20-300 ng/mL; adult females, 20-120 ng/mL; children (aged 1 month), 200-600 ng/mL; children (aged 2-5 months), 50-200 ng/mL; children (aged 6 months-15 years), 7-140 ng/mL; newborns, 25-200 ng/mL.3
Total Iron-Binding Capacity (TIBC) Test: This test measures the amount of iron that the blood would carry if the transferrin were fully saturated. Since transferrin is produced by the liver, the TIBC test can be used to monitor liver function and nutrition. Normal TIBC values are as follows: adult males, 300-400 mcg/dL; adult females, 300-450 mcg/dL. The transferrin test is a direct measurement of transferrin in the blood. The saturation level of the transferrin can be calculated by dividing the serum iron level by the TIBC. Normal transferrin values are as follows: adult males, 200-400 mg/dL; adult females, 200-400 mg/dL; children, 203-360 mg/dL; newborns, 130-275 mg/dL. Normal transferrin saturation values are between 30% and 40%.3
Candidates for Parenteral Iron
Injectable irons may be given to patients who have extremely low levels of iron or who have lost a large amount of blood. Because iron by injection does not have to be absorbed through the intestines, it is delivered directly to the circulatory system and can help build red blood cells more quickly than oral iron.4 The following patients are candidates for injectable iron treatment: patients who are receiving Epogen or Procrit (erythropoietin therapy); patients who did not tolerate oral iron or in whom supplemental iron was not effective; and patients with significant blood loss.
For patients with anemia who are receiving the drugs Epogen or Procrit, iron injections are necessary to ensure that the body has an ample and steady supply of iron. When patients are getting erythropoietin, they are building up red blood cells, which increases the need for iron. To meet increased demand, patients need to have iron available in the blood.
Patients who are most often given these drugs to stimulate the production of red blood cells are those who have anemia caused by cancer chemotherapy treatment, kidney failure, or drugs used to treat AIDS, or who are scheduled to have surgery. Some elderly patients or patients with inflammatory bowel disease or rheumatoid arthritis may also benefit from iron injections.4
Types of Parenteral Irons
Iron injections are administered either directly into the blood stream through an IV line or into the muscle. Unlike oral supplements, iron injections need to be administered by a trained professional in a clinic or hospital.
There are different types of solutions containing iron that can be injected into the circulatory system or muscle to raise iron levels in the body. The appropriate type, amount, and frequency of iron injections are determined for each individual patient based on the severity of the iron deficiency and the ability to tolerate the treatment. Iron injections comprise four major types: iron sucrose, iron dextran, sodium ferric gluconate, and ferumoxytol.
Iron Sucrose (Venofer): This form (elemental iron 20 mg/mL) is used in the treatment of iron-deficiency anemia in chronic renal failure, for patients who are not dependent on dialysis (with or without erythropoietin therapy), and for patients who are dependent on dialysis and are receiving erythropoietin therapy. Given IV, iron sucrose is less likely to trigger an allergic reaction but cannot be given in large doses. It is normally administered in a series of smaller doses over a period of days or weeks. Doses expressed in mg of elemental iron and product labeling do not indicate the need for a test dose in product-naïve patients.5
Iron Dextran: The form INFed (elemental iron, 50 mg/mL, low molecular iron) is given both IV and IM, while the form Dexferrum (elemental iron, 50 mg/mL, high molecular iron dextran) is given only IV and is administered slowly. Dexferrum can be given in a single, large dose; however, a small percentage of patients are allergic to this form of iron solution and may not be able to tolerate large doses or any size doses. An allergic reaction can cause patients to experience anaphylaxis, an anaphylactic reaction, or even cause death (as described later in the section on side effects). Normally, a small test dose is given first to determine if the patient is allergic to this form of iron solution. Both test and full doses must always be given by a health care professional.6 Dosing is as follows: dose (mL) = 0.0442 (desired hemoglobin [Hbg; usually 14.8 g/dL] minus observed Hgb) × LBW [lean body weight in kg] + (0.26 × LBW).
Sodium Ferric Gluconate (Ferrlecit): This injectable iron (elemental iron 62.5 mg/5 mL) is used for repletion of total body iron content in patients with iron-deficiency anemia who are undergoing hemodialysis in conjunction with erythropoietin therapy. The dose is 125 mg elemental iron per 10 mL (either by IV infusion or slow IV injection). Most patients will require a cumulative dose of 1 g elemental iron over approximately eight sequential dialysis treatments to achieve a favorable response. This dose is normally administered in a series of smaller doses over a period of days or weeks. Doses expressed in mg of elemental iron and product labeling do not indicate a need for a test dose in product-naïve patients.7
Ferumoxytol (Feraheme): This form (elemental iron 30 mg/mL) is given IV and is administered quickly. This iron solution can be given in similar-sized doses as iron dextran and can also be safely injected over a shorter time period. Generally, two doses are given to patients, with the second dose in 3 to 8 days. This new form of iron injection was recently approved by the FDA. Dose is as follows, for iron-deficiency anemia in chronic kidney disease—IV: 510 mg (17 mL) as a single dose, followed by a second 510-mg dose 3 to 8 days after initial dose. Recommended dose may be readministered in patients with persistent or recurrent iron deficiency anemia.8 (For an explanation of Feraheme, visit www.feraheme.com/about/
Side Effects Caused by Iron Injections
Some of the side effects are flushing, headache, muscle and joint pain, dizziness, nausea, rashes, pain and inflammation at the injection site, fever, or chills. Some patients may also experience a drop in blood pressure. Side effects may appear while the patients are receiving the injection or following completion of the injection.
A very small percentage of patients who are allergic to iron dextran can experience anaphylaxis or an anaphylactic shock. Anaphylaxis will usually bring about hives that itch and flushed or pale skin, and can include a constriction of the airway, swelling of the tongue or throat, a weak and rapid pulse, nausea, vomiting, diarrhea, dizziness, or fainting. These severe allergic reactions must be treated immediately in the emergency room or hospital. If untreated, anaphylaxis can lead to unconsciousness or death.9
Managing Iron Doses
Once the patient has started treatment, clinicians will need to closely monitor how the body reacts to the iron injections. This means they will regularly measure a patient's iron levels and Hbg levels to gauge how much iron is successfully being incorporated into the patient's red blood cells, especially during pregnancy. Depending on the status of iron levels, a health care professional may need to raise or lower the amount of solution that is received by patient and increase or decrease the number of doses each week or month.9
Oral Iron Salts and Their Elemental Iron
Among three ferrous salts, the fumarate has the highest elemental iron. A 325-mg ferrous fumarate has 108 mg iron; a 325-mg ferrous sulfate has 65 mg iron; and a 325-mg ferrous gluconate has 35 mg iron.10
These iron salts may cause some side effects, such as upset stomach and constipation or dark-colored stool. If the patient is unable to take oral iron supplements or the supplements are not effectively increasing iron levels, the clinician might choose to give one of the above iron injections. It is reported that vitamin C will increase the absorption of supplemental irons.
REFERENCES
1. ACOG Practice Bulletin No. 95: anemia in pregnancy. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;112(1):201-207.
2. Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol. 2004;15:1623-1632.
3. MedlinePlus Web site. National Institutes of Health. www.nlm.nih.gov/medlineplus/
4. Rizzo JD, Somerfield MR, Hagerty LK, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update. Blood. 2008;111(1):25-41.
5. Aronoff GR, Bennett WM, Blumenthal S, et al. Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int. 2004;66(3):1193-1198.
6. Leijn E, Monnens LA, Cornelissen EA. Intravenous iron supplementation in children on hemodialysis. J Nephrol. 2004;17(3):423-426.
7. National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2007;50(3):529-530.
8. Auerbach M. Ferumoxytol as a new, safer, easier-to-administer intravenous iron: yes or no. Am J Kidney Dis. 2008;52(5):826-829.
9. Baker WF Jr. Iron deficiency in pregnancy, obstetrics, and gynecology. Hematol Oncol Clin North Am. 2000;14(5):1061-1077.
10. Micromedex Health Series. Thomson Reuters, 2010.
To comment on this article, contact rdavidson@uspharmacist.com.






