US Pharm. 2006;7:HS-10-HS-20.
Parenteral
nutrition (PN), the provision of nutrients via the intravenous (IV) route, is
in some cases a life-saving therapy in patients who are unable to tolerate
oral or tube feedings for prolonged periods. The development of a bedside
technique for accessing a large vein (e.g., subclavian) enabled hypertonic
fluids to be administered beginning in the late 1960s, allowing a patient's
full nutritional needs to be met without the phlebitis encountered when
hypertonic fluids were administered through peripheral veins.1 This
article will address PN in adults, but many of the principles also apply to
children.
The following terms have been used in association with parenteral nutrition:
• Peripheral parenteral nutrition (PPN): The delivery of nutrients
into a small vein using a feeding catheter. • Central parenteral
nutrition (CPN): Used when the catheter tip is placed in a large, high-flow
vessel such as the superior vena cava.
• Total
parenteral nutrition (TPN): A misleading term because many patients who
currently receive nutrition by vein also concomitantly receive nutrition by
mouth or by enteral (tube) feedings.
•
Hyperalimentation: While this term is still used, it implies overfeeding
calories beyond a patient's requirements--a practice that has been largely
replaced by more conservative feeding.
Indications
PN is commonly used
in such conditions as severe pancreatitis, short-bowel syndrome, inflammatory
bowel disease exacerbations, and gastrointestinal (GI) fistulae, as well as in
critically ill patients, infants with very low birth weight, and patients with
cancer receiving hematopoietic cell transplantation.2 While enteral
nutrition (EN) may be more beneficial in some conditions (most notably, severe
pancreatitis and critical illness), PN is still commonly used.
When to initiate PN or EN
(collectively known as specialized nutrition support[SNS]) is
controversial and can dramatically impact the number of patients receiving SNS.
2 The hospital pharmacist should be aware that administration of PN is
never a medical emergency.2 Although there is evidence that
administration of EN within a few hours of severe injuries (e.g., trauma,
burns) may improve patient outcomes, no such evidence exists for PN. Both PN
and EN should be delayed until patients are hemodynamically stable (i.e., do
not require high or widely fluctuating dosages of vasopressor medications).
2
An institutional usage
pattern, in which many patients receive PN for a week or less and then
transition to adequate oral intake, should prompt the hospital pharmacist to
investigate whether prescribers are appropriately selecting patients for this
expensive, potentially dangerous therapy (see "Complications" for the dangers
of PN). Few data support improved outcomes in patients receiving
short-duration PN.2 However, patients receiving no nutrition for 10
to 14 days are likely to have poorer clinical outcomes. Current guidelines
from the American Society for Parenteral and Enteral Nutrition state that SNS,
with a preference for EN, should be initiated when oral intake has been or is
expected to be inadequate for seven to 14 days.2 A patient's
preexisting nutritional status should be taken into account, with SNS
typically started earlier in previously malnourished patients.
Access Devices
For short-term CPN
in the hospital, a temporary central venous catheter is placed percutaneously
into the subclavian vein by a physician at the bedside, with the catheter tip
at the superior vena cava adjacent to the right atrium.3 If PN
duration is expected to be more than a few weeks, a subcutaneously tunneled
catheter is placed with the tip at the superior vena cava; this procedure is
usually performed in the operative suite. With more permanent devices, such as
the Hickman catheter or Port-a-Cath, the injection port may be external or
completely beneath the skin, respectively. A peripherally inserted central
catheter (PICC) is another central venous access device that can be placed by
specially trained nurses at the bedside.4 The PICC is a central
line through which hypertonic fluids can be administered. The device is
usually inserted into the basilic vein on the inside of the elbow and threaded
so that the tip of the catheter rests at the superior vena cava.
Peripheral access for PPN is
uncommon in the United States, compared to other parts of the world.5
When PPN is used in the U.S., osmolality of the infusate is usually limited
to approximately 900 mOsm/L, and duration of therapy is limited to about seven
to 10 days. A midline catheter (i.e., a catheter placed via the basilic vein
with the tip in a vein in the upper arm) is a peripheral access device through
which fluids with osmolality above 900 mOsm/L should not be administered, due
to risk of phlebitis.
Components of PN
Components of PN
can be divided into macronutrients (i.e., protein, carbohydrate, fat) and
micronutrients (i.e., electrolytes, vitamins, trace minerals). A patient's
fluid load must also be considered when PN is administered.
Protein is provided as crystalline amino
acid solutions. Manufacturers supply standard IV amino acid products that
contain a mixture of essential amino acids (EAA) and nonessential amino acids
(NEAA), which are appropriate for most adult patients receiving PN. These
manufacturers also provide amino acid formulations that are specially designed
for young children (TABLE 1). Although the amounts of EAA and NEAA in
standard products vary slightly between manufacturers, the differences are
generally not clinically significant. However, clinically significant
differences may exist in the endogenous electrolyte content of various
products, most notably in the phosphorus, acetate, and chloride content. When
switching products due to shortages or contract changes, a brief study of
electrolyte differences is prudent.
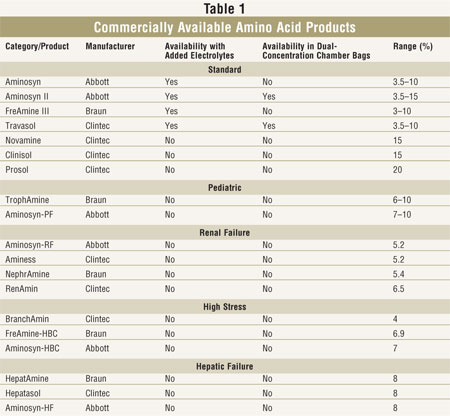
Amino acid products are
supplied in concentrations from 3.5% to 20%; more concentrated solutions are
useful in compounding for fluid-restricted patients. Amino acid formulations
are available with or without added electrolytes. Added electrolyte solutions
may be useful in institutions where PN use is minimal, as they minimize the
number of admixtures necessary. However, fixed electrolyte content may not be
appropriate for many patients, especially those who are critically ill.
Products without added electrolytes still contain some electrolytes. Amino
acid solutions provide 4 kcal/gram of amino acid.
Pediatric formulations are
commonly used in very young children. Specialty products designed for patients
with renal failure, hepatic failure, and high stress are not widely used
because they have little proven clinical benefit. Most experienced nutrition
support clinicians prefer to use less expensive standard formulations in these
populations.
Dextrose is the most common
carbohydrate used in PN solutions. Dextrose solutions commonly used for
compounding range from 10% (for PPN solutions) to 70%, with final
concentrations of dextrose commonly in the range of 5% (for PPN) to 30%.
Dextrose for IV use provides 3.4 kcal/gram. Manufacturers cannot supply
dextrose and amino acid premixed because these products react when heat
sterilized. ProcalAmine combines glycerol 3% with amino acid 3%, a mixture
that can be heat sterilized and supplied commercially. This product is used as
PPN in some institutions. If used as PPN, IV lipid should generally be
piggybacked to increase calories. Caloric density of glycerol is 4.3
kcal/gram. Although glycerol may be useful in controlling blood glucose,
especially in patients with diabetes, the low concentrations of glycerol and
amino acid in ProcalAmine limit its usefulness.
Another method used by manufacturers to
facilitate the mixture of dextrose and amino acid solutions is provision in
dual-chamber bags. To combine dextrose and amino acids, a septum between two
chambers is broken and contents are mixed. There is room to add fat emulsion
if desired. Amino acid solutions available in dual-chambers are noted in
TABLE 1. These products are supplied with and without added electrolytes.
Lipid is supplied in the U.S.
under the trade names Intralipid, Liposyn II, and Liposyn III. These soybean
oil or safflower plus soybean oil–based emulsions primarily contain the
long-chain fatty acids linoleic and linolenic acid. These products contain egg
yolk phospholipids as emulsifiers and glycerol for tonicity. IV lipid provides
1.1 kcal/mL for 10% emulsion, 2.0 kcal/mL for 20% emulsion, and 2.9 kcal/mL
for 30% emulsion. Due to concerns that long-chain triglyceride emulsions used
in the U.S. may be immunosuppressive, there is interest in alternative
emulsions.6 Alternatives containing medium-chain triglycerides and
olive oil are available in Europe and may have immunologic and metabolic
advantages.
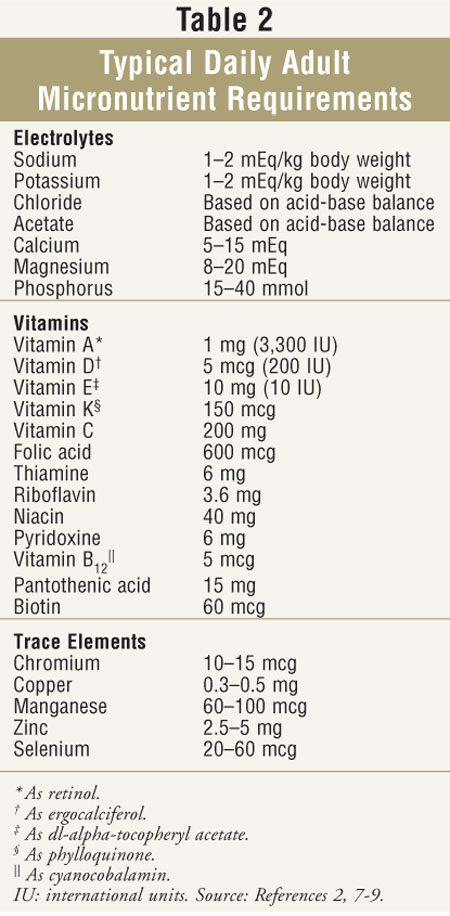
Micronutrient components of PN solutions
include electrolytes, vitamins, and trace minerals. The electrolytes usually
present include sodium, potassium, magnesium, calcium, phosphorus, chloride,
and acetate. Typical daily adult micronutrient requirements are listed in
TABLE 2.2,7-9 Requirements for predominantly intracellular
electrolytes (potassium, magnesium, and phosphorus) are somewhat driven by
carbohydrate content of the PN, with requirements increasing as carbohydrate
increases. Since these electrolytes are primarily excreted by the kidneys,
infused amounts required may be lower in patients with renal insufficiency.
Monitoring for serum electrolytes is useful for guiding the amount of
electrolyte placed in PN. It is noteworthy that serum sodium is often not
reflective of total body sodium stores, although serial values can be useful
for monitoring fluid status. Patients with metabolic alkalosis may benefit
from increasing chloride and decreasing acetate in the PN, whereas patients
with metabolic acidosis may benefit from the opposite profile of these
electrolytes. Sodium bicarbonate should not be added to PN solutions as an
alkalinizing agent because it can interact with calcium to form insoluble
calcium carbonate; sodium acetate or potassium acetate should be used instead.
9
Vitamins are usually added
using parenteral multivitamin preparations, which contain 12 or 13 essential
vitamins. The number of vitamins in most commercial preparations has recently
been reformulated based on FDA guidelines.10 The most notable
change has been the addition of vitamin K to much of the adult parenteral
multivitamin market. The 150 mcg amount of phylloquinone in a daily supply is
relatively little and should not clinically affect warfarin anticoagulation
when administered consistently. Nevertheless, the international normalized
ratio should be monitored closely in patients receiving warfarin in whom PN is
being started or discontinued. Shortages of parenteral multivitamins have
occurred in recent years; in such instances, the addition of individual
vitamin ingredients such as thiamine and folic acid may be important to avoid
complications.
Zinc, chromium, manganese, and
copper are the four trace elements most commonly added to PN solutions.
Selenium is also added, although not as universally for short-term PN
patients. Commercially available products containing a combination of trace
elements are frequently used. Some institutions add zinc in quantities beyond
those found in commercial mixtures for certain surgical patients. Copper and
manganese undergo biliary excretion and can accumulate in patients with severe
hepatic disease; they should be omitted in patients with significantly
elevated total bilirubin.2
Iodine and molybdenum are
trace elements added less frequently, usually in long-term PN. Aluminum is a
contaminant of parenteral additives that can add up to potentially unsafe
amounts in neonates and in patients with renal failure. This has prompted the
FDA to require disclosure of aluminum content of many of the parenteral
products used in compounding PN.11 Monitoring for iron deficiency
is important in long-term PN patients. Although iron is not routinely added to
PN, the mineral may be added to PN solutions containing dextrose and amino
acids, but not to solutions containing lipid emulsion due to stability issues.
Iron dextran is the form of iron most commonly added to PN.
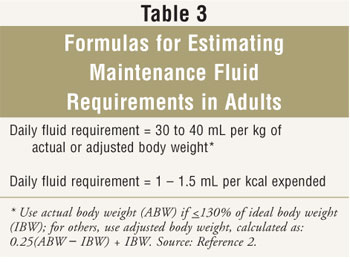
Fluid requirements for patients receiving PN
should be monitored. Daily weights are useful in hospitalized patients; weight
change of more than 0.5 kg in a day is due largely to fluid gain or loss,
rather than change in lean body mass or fat. Inputs and outputs should be
monitored in acute care to gauge fluid status. Serial monitoring of blood for
albumin, sodium, and hematocrit may also be helpful in determining fluid
status when used in combination with body weight and inputs and outputs; these
values can reflect dilution and concentration.
Formulas
for estimating maintenance fluid requirements in patients without unusual
losses are found in TABLE 3.
Compatibility and Stability Issues
Calcium and phosphate solubility is
a major issue concerning the compatibility of PN formulations. Solubility is
influenced by several factors such as temperature; calcium phosphate
solubility decreases with increasing temperature.12 Formulations
that appear stable when refrigerated could form precipitates at room
temperature. Another important factor is pH; calcium phosphate solubility
increases as pH decreases. Higher final amino acid and dextrose concentrations
are associated with lower pH and thus higher calcium phosphate solubility.
Calcium gluconate is preferred
in PN solutions due to superior solubility compared to calcium chloride. The
order in which calcium and phosphate are added is important; phosphate is
generally added first, while calcium is added near the end of the compounding
sequence. The amounts of calcium and phosphate added must be considered, with
a greater chance of precipitation if the amount of one or both is increased
above standard. If lipid is admixed with the PN to form a total nutrient
admixture (TNA), visual detection of calcium phosphate precipitates becomes
more difficult. The pharmacist must follow the manufacturer's calcium and
phosphate guidelines for specific products and concentrations comprising any
PN admixture. Simplified formulas for estimating the maximum amount of calcium
and phosphate that can be placed in PN formulas are fraught with error.
In-line, 0.22-micron (preferred), or 1.2-micron filters should be used when
infusing PN solutions containing dextrose plus amino acid.9 TNA
should be infused through a 1.2-micron filter.9
TNA poses greater challenges
in terms of stability due to the lipid component, as compared to dextrose plus
amino acid solutions. Chemical stability can be compromised by excessive
cations, particularly divalent cations, resulting in "creaming" or "cracking"
of the TNA. With creaming, lipid can be redispersed with gentle inversion and
administered to a patient.9 However, with a cracked TNA, separated
lipid does not redisperse with gentle inversion and must not be administered.
9 For maximal stability, TNA should contain final concentrations of
macronutrients within the following ranges: dextrose, 3.3% to 35%; amino acid,
1.75% to 5%; and lipid, 2% to 6.7%.8
Pharmacists should also
consider the expiration time for IV lipids hung separately from the dextrose
and amino acid. A TNA is generally considered microbiologically safe for 24
hours after initial hanging. However, lipid emulsion alone is a better growth
medium due to its nearly physiologic osmolality and pH. This is in contrast
with a TNA that is hypertonic and has a lower pH. The current CDC
recommendation is that a lipid emulsion hung alone should not infuse for more
than 12 hours after spiking the container.13 Literature support for
this recommendation has been summarized elsewhere.14
Nutritional Assessment
Assessing the quantitative needs of
patients receiving PN is important. Overfeeding macronutrients or
micronutrients can lead to complications, while underfeeding can be associated
with malnutrition or micronutrient deficiency. Assessment of nutritional
status has historically been performed based on a combination of physical
examination characteristics, biochemical parameters, and immunological
markers. Immunological markers include total lymphocyte counts and anergy
screening. Unfortunately, these markers are nonspecific and have largely been
abandoned as nutritional markers.
Widely used biochemical
markers include serum albumin and other circulating proteins. Albumin
concentrations fluctuate based on hydration status and can drop precipitously
following stress or injury as protein redistributes. The long half-life of
albumin (about 21 days) does not make it optimal for serial monitoring in
hospitalized patients, although it is often a good marker of long-term
nutritional status. Therefore, shorter half-life proteins are frequently used
for tracking nutritional response to feeding. Prealbumin is perhaps most
commonly used (half-life is about two days). In critically ill patients,
prealbumin concentrations are sometimes used with C-reactive protein (CRP)
concentrations. CRP is an acute phase reactant and marker of inflammation.
Synthesis of prealbumin is not a priority of a stressed patient's body until
inflammation begins to decline. Therefore, a significant rise in prealbumin is
not expected--even with adequate nutritional support--until CRP declines.
Prealbumin can be affected by conditions other than malnutrition, such as
renal and hepatic disease.
Early in the PN era,
measurements such as mid-arm muscle circumference and skin folds of the
triceps were widely used to help determine nutritional status. These methods
are now rarely used in the clinical setting. More commonly used is the
subjective global assessment technique, which considers recent changes in
weight and dietary intake, presence of GI symptoms, functional capacity, and
concomitant diseases.15
Indirect calorimetry (IC) is
the gold standard clinical tool for determining calorie requirements of SNS
patients. IC measures carbon dioxide production and oxygen consumption.
Resting energy expenditure (REE) is calculated from these values. Patients are
fitted with a mask or mouthpiece, or a rigid canopy is placed over their head.
Patients receiving mechanical ventilation can have IC performed by hooking
into the ventilatory apparatus. Recently, less expensive hand-held IC devices
have been marketed, which may be useful for alert patients who can cooperate
with measurement, although this is often not the case in hospitalized
patients.
The REE obtained from IC is a
guide for determining how many calories to feed. Typically, hospitalized
patients are fed near their REE, although sometimes they are fed well below
their REE (permissive underfeeding). Permissive underfeeding may be
particularly useful in morbidly obese patients; the optimal amount of calories
for this population is still being investigated.16 The maximum
amount of dextrose recommended in adult PN is 7 g/kg/day, and maximum lipid
amount is 2.5 g/kg/day.9 However, these maximums are rarely
approached in current clinical practice. Dextrose is typically supplied at 3
to 5 g/kg/day, while lipid is often limited to less than 1 g/kg/day in
critically ill and immunocompromised patients.
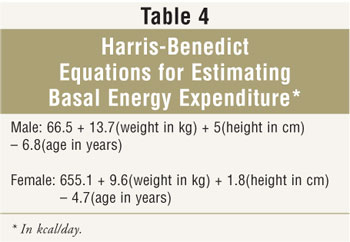
Since many institutions and home care
agencies do not perform IC, caloric requirements must be estimated. Many
clinicians use Harris-Benedict equations to estimate basal energy expenditure
(BEE) (TABLE 4). Activity level and/or stress factors are often added
to calculated BEE, which sometimes results in overfeeding. Other formulas,
such as the Swinamer and Frankenfield equations, have been developed for
specific populations. Alternatively, many clinicians estimate caloric
requirements on a kcal/kg basis; typical ranges provided by this approach are
20 to 30 kcal/kg/day. Determining which weight to use to calculate caloric
requirements in obese patients is controversial. Many clinicians use an
"adjusted body weight," such as ideal body weight plus about 25% to 50% of
excess weight.17
Providing adequate protein is
important when formulating PN. In fluid-restricted patients, it is sometimes
necessary to choose between goal calories or goal protein. In such a
situation, many clinicians would choose to meet goal protein requirements at
the expense of goal energy requirements. Typically, patients receiving PN are
given 1 to 2 g of protein per kg of body weight per day. In general, the more
highly stressed a patient is, the more protein he or she requires to maintain
nitrogen equilibrium (i.e., to prevent lean body mass loss). In patients
weighing less than ideal body weight, actual body weight should be used to
calculate caloric and protein requirements. In obese patients, adjusted body
weight is commonly used to determine protein requirements.
A nitrogen balance study can
estimate whether SNS is meeting a patient's protein requirements. A 24-hour
urine collection is performed and urinary urea nitrogen (UUN) or total urea
nitrogen (TUN) is measured by the laboratory. Although TUN is preferable, UUN
is more commonly measured because it is easier for the laboratory to perform.
The formula for calculating nitrogen balance when UUN (in g/day) is reported
is:
Nitrogen balance = Protein intake (g) –
(UUN + 4)
6.25
The number 4 in this formula is an estimate of
fecal and cutaneous loss of nitrogen (2 g), plus non-urea urinary nitrogen (2
g). To calculate nitrogen intake, the number of grams of protein supplied to
the patient is divided by 6.25. Nitrogen makes up about 16% of the total
weight of amino acids in commercially available IV products. The goal is to
have a positive balance; that is, it is preferable that a patient receive more
nitrogen than is excreted, which implies a net gain of lean body mass.
However, this is unrealistic for many severely ill patients during the height
of disease. In such cases, the goal is to minimize the loss of lean body mass
(i.e., minimize the negative nitrogen balance as much as possible).
Certain patients may require protein in
amounts greater or less than 1 to 2 g/kg. Patients with renal insufficiency in
whom dialysis has not been initiated may not tolerate protein at 1 g/kg.
However, protein in lower amounts is not optimal because acute renal
insufficiency is most frequently seen concomitantly with catabolic illnesses.
Such patients require dialysis in order to be adequately fed from both a fluid
and protein standpoint. Dialysis therapy also removes excess nitrogenous waste
from protein metabolism. Patients receiving some of the newer continuous renal
replacement therapies (CRRTs) may benefit from more than 2 g/kg due to large
protein losses with CRRT.18 Patients with end-stage liver disease
may need to have protein restricted to less than 1 g/kg in the presence of
hepatic encephalopathy.
Complications
Complications of PN can be divided
into three main categories--mechanical, metabolic, and infectious. Mechanical
complications include pneumothorax with catheter placement, thrombosis, and
phlebitis. A chest x-ray should always be performed after catheter insertion
to ensure that the catheter tip is correctly located before PN administration.
Thrombosis can occur at the catheter tip and generally begins with formation
of a fibrin sheath on the outside of the catheter. Clearing of a catheter
occlusion due to a fibrin sheath or thrombosis can be accomplished by infusion
of a thrombolytic agent, such as tissue plasminogen activator, through the
catheter.19 Some patients with permanent central catheters who
receive home PN are given low-dose warfarin to help prevent thrombosis;
efficacy of this technique is debated, and more evidence supports this
practice in patients with malignancies than in patients receiving home PN.
20,21 The addition of heparin to PN does not appear to decrease
thrombosis risk.20
Thrombophlebitis is a limiting
complication of PPN. Phlebitis with PPN can be minimized through frequent
rotation of catheter sites and careful choice of catheter size and type.
5,22 A commonly cited recommendation is to limit osmolality of PPN to
less than 900 mOsm/L; recommendations for both lower and higher limits of
osmolality are found in the literature.5,22 It appears that PPN
formulated as TNA is better tolerated than dextrose/amino acid mixtures with
lipid piggybacked into the IV line, regardless of osmolalities. The addition
of heparin and hydrocortisone to PPN solutions has not been effectively shown
to reduce phlebitis.5
Electrolyte abnormalities are
metabolic complications of PN. Significant preexisting abnormalities are
preferably corrected prior to PN initiation. Hypokalemia,
hypomagnesemia, and hypophosphatemia are common complications of PN. Adding
more of these electrolytes to the PN or as separate infusions should correct
these abnormalities. Hyperkalemia, hypermagnesemia, and hyperphosphatemia are
most commonly seen with renal insufficiency; restriction should help correct
these abnormalities. Alteration of the acetate-to-chloride ratio may be
helpful in correcting metabolic acidosis or metabolic alkalosis that may or
may not be related to PN. Specific guidelines for the correction of
electrolyte abnormalities in critically ill patients have been published.
23
Vitamin and trace element
deficiencies can occur during long-term PN. Some home care companies may
monitor serum concentrations of certain micronutrients on a regular basis,
perhaps once or twice a year.24 Specific patient parameters may
prompt the clinician to monitor a certain micronutrient. For example, patients
with draining fistulas may be monitored closely for development of zinc
deficiency. Concern about accumulation of copper and manganese in patients
with significant hepatic disease is prudent; in such cases, these trace
elements may be omitted, and chromium, zinc, and selenium may be added as
separate entities. Generally, monitoring for vitamin and trace element
abnormalities becomes more critical as a patient remains on PN for a longer
amount of time.
Overhydration and dehydration
are concerns in patients receiving PN. The pharmacist is frequently called
upon to concentrate or dilute PN to better match fluid requirements.
The importance of tight
glycemic control, especially in critically ill patients, has recently been
emphasized.25 Starting with a low amount of dextrose in the PN
(less than 2 g/kg/day) and titrating up to goal rate (usually 3 to 5 g/kg
depending on caloric requirements) over several days may be helpful in
preventing extreme glycemic excursions. Many patients will require insulin to
keep blood glucose within acceptable limits. Insulin should be added to PN in
the pharmacy preparation area; it should not be added after the PN is hung,
due to sterility concerns. One recommendation is to start with 0.1 unit of
insulin per gram of dextrose in the PN container and increase in increments of
0.05 unit per gram, with subsequent mixes as necessary.26 For
patients with more extreme increases in blood glucose, a separate insulin drip
is preferred to fine-tune the insulin. Many clinicians now strive to keep
blood glucose levels as close to normal as possible in critically ill patients
and below about 150 mg/dL in hospitalized patients who are less severely ill.
26
Gross overfeeding can lead to
excessive carbon dioxide production and could interfere with weaning from
mechanical ventilation. Since metabolism of carbohydrate results in production
of more carbon dioxide than metabolism of lipid, it was sometimes recommended
to give relatively more lipid and less dextrose in mechanically ventilated
patients.27 With lower numbers of total calories currently
recommended, this is probably not clinically relevant.
Liver function test
abnormalities have been frequently reported in patients receiving PN. These
abnormalities are generally divided into two categories in adult
patients--hepatic steatosis and cholestasis.28 Hepatic
steatosis, or fat accumulation in the liver, is manifested as an elevation of
aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Hepatic
steatosis due to PN is not as common as in the past, due to conservative
amounts of nutrients now prescribed. However, elevations in ALT and
AST--especially in the first seven to 10 days of PN--should cause the clinician
to reassess the formulation to ensure the patient is not being overfed.
Most patients on long-term PN
develop some cholestasis. In the absence of enteral intake, the gallbladder is
not stimulated to empty. Bile becomes thick and sludgy and can eventually
cause biliary obstruction. Elevations in total bilirubin and alkaline
phosphatase occurring a few weeks or more after initiation of PN may indicate
cholestasis. The best prevention and treatment is the use of enteral feedings
(even small amounts), if possible.
Metabolic bone disease is a
complication unique to home PN. Many patients receiving long-term PN will
develop osteoporosis or osteomalacia. The definitive cause is unknown,
although several preventative strategies such as careful attention to the
amounts of calcium, magnesium, phosphorus, and vitamin D provided in the PN
have been suggested.29 Limitation of protein in the PN to about 1
g/kg/day in the long-term patient may also help prevent hypercalciuria, thus
preserving bone mass.29
Catheter-related sepsis (CRS)
is the most common cause of hospitalization in home PN patients. CRS can also
be a complication of patients receiving PN through a temporary access device.
With temporary devices, the catheter is typically replaced if infection is
suspected. With permanent devices, attempts to salvage the catheter are often
made because of difficulty in removing and replacing the device.30
In these cases, systemic antibiotic therapy is attempted if the patient is not
seriously ill. The catheter is removed and replaced only if infection fails to
clear after an adequate trial of antibiotics. Most clinicians would remove the
catheter if fungal CRS is confirmed, as this is exceedingly difficult to clear
with the catheter in place.
Monitoring
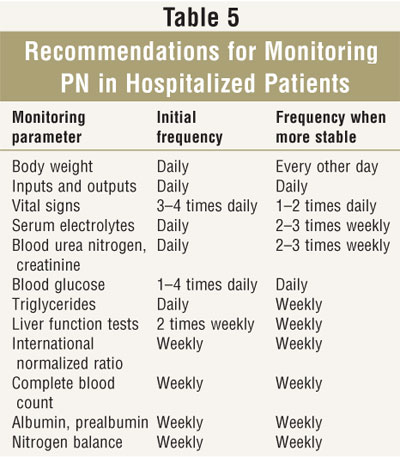
General recommendations for monitoring PN are listed in TABLE 5. Monitoring should be individualized, and baseline values should be obtained for most of these parameters prior to PN initiation. In critically ill patients, monitoring is generally performed more frequently than in stable patients. Laboratory monitoring may be done quite infrequently in stable patients on home PN.
Drug Compatibility with PN
Several drugs have been proven stable when admixed
with PN solutions and are commonly added. The most common are histamine-2
antagonists and regular insulin. Iron dextran is also sometimes added to
dextrose/amino acid mixtures but is incompatible with TNA. In addition,
pharmacists are often queried regarding Y-site compatibility of various drugs
with PN solutions. The reader is referred to a standard reference text for
information regarding compatibility of drugs with PN solutions.12
Conclusion
PN, a potentially lifesaving
therapy, is sometimes combined with intake via the oral or tube route. Some
physicians still use PN in situations where no SNS is required, such as in
previously adequately nourished patients who are expected to resume oral
intake within a week. Other physicians underuse EN and instead prescribe PN in
patients with a functional gut. In patients requiring PN, the pharmacist will
be called upon for expertise, especially when stability and compatibility
issues arise. While the amount of dextrose and lipid supplied in PN has
decreased over the years, the value of supplying substantial protein is still
recognized. Since parenteral micronutrient requirements are sometimes
difficult to determine, PN requires careful monitoring. The emerging
importance of tight glycemic control in hospitalized patients is another
challenge for clinicians managing PN.
REFERENCES
1. Dudrick SJ. A 45-year obsession
and passionate pursuit of optimal nutrition support: puppies, pediatrics,
surgery, geriatrics, home TPN, A.S.P.E.N., et cetera. J Parenter Enteral
Nutr. 2005;29:272-287.
2. A.S.P.E.N. Board of
Directors. Guidelines for the use of parenteral and enteral nutrition in adult
and pediatric patients. J Parenter Enteral Nutr. 2002;26(1 Suppl)
1SA-138SA.
3. Grant JP. Parenteral
access. In: Rombeau JL, Rolandelli RH, eds. Clinical Nutrition: Parenteral
Nutrition. 3rd ed. Philadelphia: WB Saunders Company; 2001:109-117.
4. Orr ME. The
peripherally inserted central catheter: what are the current indications for
its use? Nutr Clin Pract. 2002;17:99-104.
5. Culebras JM,
Garcia-de-Lorenzo A, Zarazaga A, et al. Peripheral parenteral nutrition. In:
Rombeau JL, Rolandelli RH, eds. Clinical Nutrition: Parenteral Nutrition
. 3rd ed. Philadelphia: WB Saunders Company; 2001:580-587.
6. Driscoll DF, Adolph
M, Bistrian BR. Lipid emulsions in parenteral nutrition. In: Rombeau JL,
Rolandelli RH, eds. Parenteral Nutrition. 3rd ed. Philadelphia: WB
Saunders Company; 2001:35-59.
7. Holcombe BJ,
Gervasio JM. Adult parenteral nutrition. In: Koda-Kimble MA, Young LY, Kradjan
WA, et al., eds. Applied Therapeutics: The Clinical Use of Drugs. 8th
ed. Philadelphia: Lippincott Williams & Wilkins; 2005;37-1–37-23.
8. Mirtallo JM.
Parenteral formulas. In: Rombeau JL, Rolandelli RH, eds. Parenteral
Nutrition. 3rd ed. Philadelphia: WB Saunders Company; 2001:118-139.
9. Task force for the
revision of safe practices for parenteral nutrition. Safe practices for
parenteral nutrition. J Parenter Enteral Nutr. 2004;28:S39-S70.
10. Parenteral
multivitamin products. Federal Register. April 20, 2000;65:21200-21201.
11. Klein GL. Aluminum
contamination of parenteral nutrition solutions and its impact on the
pediatric patient. Nutr Clin Pract. 2003;18:302-307.
12. Trissel LA.
Handbook on Injectable Drugs. 13th ed. Bethesda, MD: American Society of
Health-System Pharmacists; 2005.
13. O'Grady NP,
Alexander M, Dellinger EP, et al. Guidelines for the prevention of
intravascular catheter-related infections. MMWR. 2002;51(RR-10):1-26.
14. Sacks GS, Driscoll
DF. Does lipid hang time make a difference? Time is of the essence. Nutr
Clin Pract. 2002;17:284-290.
15. Detsky AS,
McLaughlin JR, Baker JP, et al. What is subjective global assessment of
nutritional status? J Parenter Enteral Nutr. 1987;11:8-13.
16. Dickerson RN.
Specialized nutrition support in the hospitalized obese patient. Nutr Clin
Pract. 2004;19:245-254.
17. Krenitsky J.
Adjusted body weight, pro: evidence to support the use of adjusted body weight
in calculating calorie requirements. Nutr Clin Pract. 2005;20:468-473.
18. Wooley JA, Btaiche
IF, Good KL. Metabolic and nutritional aspects of acute renal failure in
critically ill patients requiring continuous renal replacement therapy.
Nutr Clin Pract. 2005;20:176-191.
19. Timoney JP, Malkin
MG, Leone DM, et al. Safe and cost effective use of alteplase for the
clearance of occluded central venous access devices. J Clin Oncol.
2002;20:1918-1922.
20. Couban S, Goodyear
M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin
for the prevention of central venous catheter-associated thrombosis in
patients with cancer. J Clin Oncol. 2005;20:4063-4069.
21. Klerk CP,
Smorenburg SM, Buller HR. Thrombosis prophylaxis in patient populations with a
central venous catheter: a systematic review. Arch Intern Med.
2003;163:1913-1921.
22. Anderson AD, Palmer
D, MacFie J. Peripheral parenteral nutrition. Br J Surg.
2003;90:1048-1054.
23. Kraft MD, Btaiche
IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients
in the intensive care unit. Am J Health Syst Pharm. 2005;62:1663-1682.
24. Fessler TA. Trace
element monitoring and therapy for adult patients receiving long-term total
parenteral nutrition. Pract Gastroenterol.
2005;44:51-52,54,56,58,60,63-65.
25. van den Berghe G,
Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill
patients. N Engl J Med. 2001;345:1359-1367.
26. McMahon MM.
Management of parenteral nutrition in acutely ill patients with hyperglycemia.
Nutr Clin Pract. 2004;19:120-128.
27. Talpers SS,
Romberger DJ, Bunce SB, Pingleton SK. Nutritionally associated increased
carbon dioxide production. Excess total calories vs high proportion of
carbohydrate calories. Chest. 1992;102:551-555.
28. Buchman A. Total
parenteral nutrition-associated liver disease. J Parenter Enteral Nutr.
2002;26(5 Suppl):S43-S48.
29. Seidner DL.
Parenteral nutrition-associated metabolic bone disease. J Parenter Enteral
Nutr. 2002;26:S37-S42.
30. Mermel LA, Farr BM,
Sherertz RJ, et al. Guidelines for the management of intravascular
catheter-related infections. Clin Infect Dis. 2001;32:1249-1272.
To comment on this article, contact
editor@uspharmacist.com.






