US Pharm. 2009;34(3)(Oncol suppl):3-10.
ABSTRACT: Colon cancer is the fourth most common cancer and second most common cause of cancer death in the United States. Colon cancer develops from small growths called polyps that protrude from the intestinal wall and undergo mutational changes that transform them into malignant tumors. Treatment options for colon cancer vary by the severity of the disease as well as individual patient characteristics but may include surgery, radiation, and/or chemotherapy. Many chemotherapy agents from different pharmacological classes have been developed and evaluated for their benefit in the management of colon cancer. Traditional chemotherapeutic agents such as fluorouracil, irinotecan, capecitabine, and oxaliplatin may be used alone or in various combination regimens. Newer agents such as cetuximab, panitumumab, and bevacizumab target specific growth-related proteins that are used by the tumors. Newer agents continue to be developed, and these offer clinicians and patients additional options for managing colon cancer.
It is estimated that 112,340 individuals were diagnosed with colon cancer in 2007, split almost evenly amongst men and women.1 The combined deaths attributed to both colon cancer and rectal cancer in that year were 52,180. These figures represent colon cancer as the fourth most common cancer--behind prostate, breast, and lung cancers--and the second most common cause of cancer-related death after lung cancer. On a positive note, incidence and disease-related mortality have declined over the past 20 years due to improved screening methods and treatment.1 Treatment is dependent upon the stage of cancer at the time of diagnosis and individual patient factors and may involve surgery, chemotherapy, and/or radiation. The discussion that follows will review the use of common pharmacological agents in the management of colon cancer.
Risk Factors and Diagnostic Screening
The National Comprehensive Cancer Network (NCCN) details factors to consider in order to establish an individual's stratified risk of developing colon cancer into categories of average risk, increased risk, and hereditary high risk.2 See TABLE 1 for a summary of these risks and classifications.
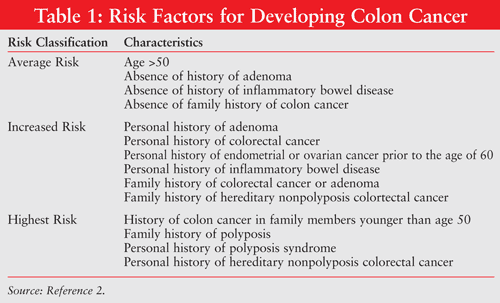
Methods used to screen for colon cancer depend on the patient's risk classification. Patients with average risk may be screened using colonoscopy, fecal occult blood test, or double-contrast barium enema.2 Patients at an increased risk for colon cancer should be screened by colonoscopy. Patients in the highest risk category require a detailed medical history of affected family members in addition to colonoscopy.2
Pathophysiology
The colon and rectum are divided into eight areas with lymph nodes interspersed along the major blood vessels that supply and are adjacent to these areas.3 The intestinal wall is made up of several layers of tissue, any or all of which could be involved in a developing tumor. Overall involvement is determined through evaluation of biopsied specimens and is used to determine the pathologic stage.3
Most colorectal cancers arise from polyps, which begin as small protrusions from the mucosal surface and may be either malignant or benign.4 A polyp is classified as a nonneoplastic hamartoma (juvenile polyp), a hyperplastic mucosal proliferation (hyperplastic polyp), or an adenomatous polyp. Only the last type is premalignant, but even so, less than 1% develop into a tumor. The incidence of adenomatous polyps increases with age, with 30% of middle-aged adults and up to 50% of older individuals having these lesions. Adenomatous polyps may be pedunculated (stalked) or sessile (flat), with the sessile type more often associated with a cancer. These polyps usually produce no symptoms, but occult fecal blood is detected in approximately 5% of patients. To become malignant, polyps pass through various stages of genetic alteration that affect the proliferative nature of the growth. It is believed that at least five years are required for a malignant polyp to become clinically significant.4 Once the tumor has begun to grow, metastases from colon cancers can travel to almost any organ in the body, but the liver and lungs are the most commonly affected.3
Staging
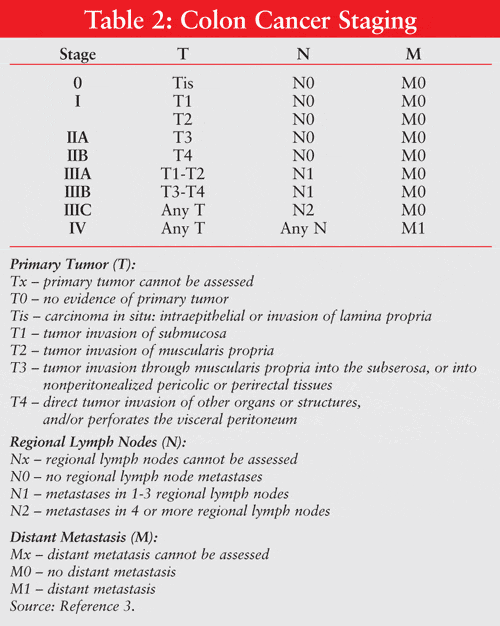
The tumor, node, metastasis method has been employed by the American Joint Committee on Cancer to categorize the staging of colon cancer (see TABLE 2). 3 This staging system classifies disease severity based on the extent of cancer spread as determined through a detailed physical exam, radiographic imaging, and surgical exploration. Staging is also important in treatment selection. An alternative staging system, used only in colon cancer, is the Dukes classification. This system is named for its developer, Cuthbert Dukes, a Scottish pathologist.5 Stage A refers to tumors that are restricted to, but not through, the bowel wall, while stages B, C, and D represent advancing tumor spread into surrounding lymph nodes and, ultimately, distant metastases. Despite revisions, this system is still limited by the absence of consideration of histology, the number of lymph nodes involved, and the depth of tumor penetration into the colonic wall.5
Treatment
The treatment for colon cancer is complex and depends upon the results of pathologic examination that occurs following surgical inspection of the affected area and determination of cancer spread.6 The initial treatment effort is surgical resection, if possible. Cancers deemed to be in stage 0 or stage I are likely to receive no initial additional medical treatment beyond surgery. Patients in stages IIA and IIB may receive adjuvant chemotherapy or be observed closely following surgical resection. Patients with any type of stage III disease should be offered adjuvant chemotherapy following surgery. All patients are subsequently monitored over the next five years following these initial treatments to survey for abnormalities suggesting the possible return of cancer.6
Patients with stage IV disease have proven metastases and therefore require a consideration of more complex treatment options based on the location of the metastases and their degree of resectability.6 They may initially receive neoadjuvant chemotherapy followed by surgical resection of the colon and metastases, followed by adjuvant chemotherapy and resection of metastatic disease. Those patients in whom the primary tumor is initially unresectable may be offered palliative therapy due to the advanced nature of their disease.6
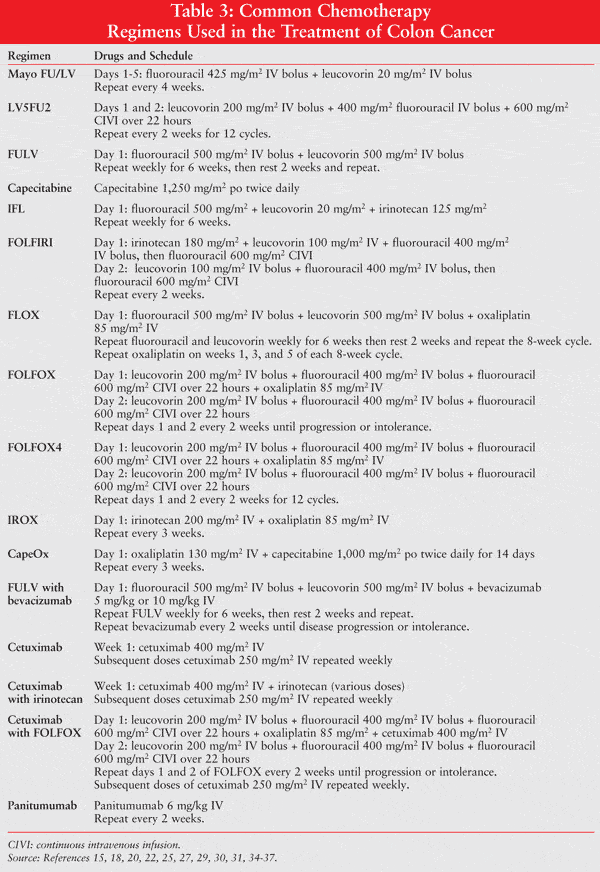
Several chemotherapy regimens are utilized to provide treatment of the various stages of colon cancer. Common drugs used in various combinations include capecitabine, fluorouracil/leucovorin, oxaliplatin, irinotecan, and bevacizumab. Other agents are also available, but their use is not described in current guidelines. Examples of combinations of recommended agents include fluorouracil with leucovorin; capecitabine plus oxaliplatin (CapeOX); fluorouracil/leucovorin plus oxaliplatin (FOLFOX); fluorouracil/leucovorin plus irinotecan (FOLFIRI); capecitabine plus oxaliplatin with bevacizumab; fluorouracil/leucovorin with bevacizumab; and fluorouracil/leucovorin plus irinotecan with bevacizumab.6 The remaining portion of this article will discuss the application of these agents.
Chemotherapy
Adjuvant chemotherapy is an attempt to destroy any residual microscopic metastatic disease that may be present following surgical resection in order to reduce the likelihood of tumor recurrence.7 The NCCN guidelines recommend the consideration of capecitabine alone, fluorouracil/leucovorin, or fluorouracil/leucovorin plus oxaliplatin as adjuvant therapy in applicable patients with stage II or stage III colon cancer.6 In the case of patients with metastatic disease, FOLFIRI, FOLFOX, or CapeOX with or without bevacizumab should be considered for neoadjuvant and/or adjuvant therapy.
Fluorouracil and Leucovorin: Fluorouracil (5-FU) is an antimetabolite antineoplastic agent formed by halogenating the ribonucleic acid (RNA) base uracil with a fluorine atom.8 After administration, fluorouracil is converted via one of two pathways to its active cytotoxic form. One pathway utilizes the enzyme uridine phosphorylase to convert 5-FU to fluorouridine and then to the active form of floxuridine monophosphate (FUMP) by the enzyme uridine kinase. The other pathway utilizes the enzyme orotate phosphoribosyl transferase to form the FUMP. Once formed, FUMP can enter several metabolic pathways to disrupt normal cellular functions involving RNA and/or deoxyribonucleic acid (DNA) synthesis.8In normal pyrimidine synthetic pathways, uracil is converted to thymidine triphosphate (TTP) as a precursor prior to incorporation into DNA.8 In an alternative mechanism of 5-FU, the insertion of the fluorine atom in 5-FU alters molecular interactions and disrupts the activity of thymidylate synthase used to create the normal components required for DNA synthesis. The presence of 5,10-methylene tetrahydrofolate (i.e., folinic acid, leucovorin) acts as a cofactor to tighten the binding of fluorouracil to thymidylate synthase into a locked formation that enhances 5-FU cytotoxicity. The result is that FdUTP and dUTP get inserted into DNA instead of TTP. This abnormal insertion results in DNA strand breakages that render the molecule useless.8
Several doses, schedules, and administration techniques using the combination of fluorouracil and leucovorin have been utilized. For example, Petrelli et al showed that the addition of leucovorin as a high dose (500 mg/m2) or low dose (25 mg/m2) to fluorouracil increased the likelihood of treatment responses in patients with metastatic colorectal cancer.9 Interestingly, there was no difference in tumor response between the two leucovorin-containing regimens.9 This study was the basis for additional evaluation and widespread use of leucovorin in combination with fluorouracil.
Other regimens utilizing fluorouracil and leucovorin have varied the doses of both drugs as well as the duration of infusions and schedule of administration.10,11 Common side effects (>20%) of fluorouracil (Adrucil) include stomatitis, esophagopharyngitis, diarrhea, anorexia, nausea, vomiting, alopecia, dermatitis, and leukopenia.12 Leucovorin is available in an oral tablet formulation as well as a parenteral form and may also be referred to as leucovorin calcium, folinic acid, or citrovorum factor.8,13 Common adverse effects seen with the use of leucovorin alone may include allergic-type reactions.13
Capecitabine: Capecitabine is an oral prodrug that is converted in vivo to 5-FU.8,14 Once ingested, capecitabine is converted through hepatic and nonhepatic mechanisms to 5'-deoxy-5-fluorocytidine, then to 5'-deoxy-5-fluorodeoxyuridine, and ultimately to 5-FU by the enzyme thymidine phosphorylase. This 5-FU then follows the same antimetabolic pathway described previously.8,14Capecitabine (Xeloda) is FDA approved for use as monotherapy in the treatment of patients with Dukes C colon cancer after surgery to remove the primary tumor.14 Dosing of capecitabine is 1,250 mg/m2 orally twice daily for two weeks followed by a one-week rest period and continuing in this repeating pattern for a total of eight cycles. The tablets should be swallowed with water within 30 minutes of a meal. Individualized patient doses should be rounded to a proper amount that allows the total dose to equal full tablet sizes. Common side effects seen with use of capecitabine include diarrhea, nausea, vomiting, stomatitis, abdominal pain, hand-foot syndrome, dermatitis, fatigue, anemia, hyperbilirubinemia, and decreased appetite.14
Although capecitabine offers the same mechanism as fluorouracil, it provides the flexibility of oral dosing. Studies have been completed to compare the two drugs. For example, Twelves et al compared the activity of each drug in patients with stage III colon cancer.15 Patients who were randomized received oral capecitabine or leucovorin plus fluorouracil. With regard to disease-free survival, capecitabine was shown to be at least as effective as fluorouracil plus leucovorin, with no difference in three-year survival between the two groups. Furthermore, the probability of relapse-free survival was determined to be statistically longer in the patients receiving capecitabine, while the three-year relapse-free survival and overall survival were no different.15 Of note is that patients receiving capecitabine developed significantly fewer toxic side effects such as diarrhea, nausea, vomiting, stomatitis, alopecia, and neutropenia. Hand-foot syndrome did occur far more often in patients receiving capecitabine.16
Oxaliplatin: Oxaliplatin is the only platinum agent currently approved for use in the treatment of colon cancer.8,17 Oxaliplatin (Eloxatin) undergoes nonenzymatic cleavage to reactive alkylating species that form inter- and intrastrand DNA crosslinks that inhibit DNA transcription and replication.17 Enhanced anticancer activity when used in combination with fluorouracil and leucovorin has lead to oxaliplatin's approval for use as adjuvant therapy in the treatment of patients with stage III colon cancer following complete resection of the primary tumor, as well as patients with advanced colon cancer. Oxaliplatin 85 mg/m2 is recommended to be given intravenously over two hours at the same time as leucovorin 200 mg/m2, in separately prepared fluid bags. This is followed by a bolus dose of fluorouracil 400 mg/m2 over two to four minutes, then a continuous intravenous infusion of fluorouracil 600 mg/m2 over 22 hours. The leucovorin and fluorouracil doses are then repeated on day 2 of this regimen. The entire three-drug regimen is re-administered every two weeks for a total of 12 cycles. Common adverse effects seen with oxaliplatin use in combination with fluorouracil and leucovorin include dyspnea, fatigue, diarrhea, constipation, nausea, vomiting, stomatitis, abdominal pain, anorexia, fever, and neuropathy.17The efficacy of oxaliplatin in combination with fluorouracil and leucovorin has been evaluated.18 Andre et al randomized patients with stage II or stage III colon cancer to receive leucovorin followed by fluorouracil on two consecutive days every 14 days for up to 12 cycles or the same regimen plus oxaliplatin on day one. The probability of disease-free survival was higher in patients receiving triple-drug therapy than in the fluorouracil arm, with a significantly lower risk of recurrence as well.18 The results of this study suggest that oxaliplatin may offer an advantage in reducing disease recurrence, but overall, adding oxaliplatin to fluorouracil and leucovorin may not significantly change survival.
Irinotecan: Irinotecan (Camptosar) is a member of the class of antineoplastics referred to as camptothecins, which act by inhibiting the enzyme topoisomerase I.8 Topoisomerase I functions by interacting with DNA and reducing the torsional stress of the supercoiled molecule. The inhibition of the enzyme occurs when camptothecins bind to the topoisomerase I/DNA complex and block repair of DNA single-strand breaks created to relieve the coiling. When normal replication processes encounter the cleaved DNA, a double-strand break is created that cannot be properly repaired, and subsequent cell death ensues.8 The active form of irinotecan is a hepatically derived metabolite known as SN-38. Genetic variations in the general population regarding metabolism of SN-38 by the enzyme uridine diphosphate-gluocuronosyltransferase create an inversely proportional risk of severe diarrhea following administration of the drug. Use of loperamide 4 mg at the onset of diarrhea, then 2 mg every two hours may help control this drug-limiting toxicity.8
Camptosar is FDA approved for use as first-line treatment in combination with fluorouracil and leucovorin in patients with metastatic colon cancer whose disease has recurred or progressed following previous fluorouracil-based treatment.19 The dosage of weekly irinotecan varies with the particular regimen but can range from 125 mg/m2 to 180 mg/m2 when used in combination regimens. When used alone, a weekly dose of 125 mg/m2 can be used or a dose of 350 mg/m2 given every three weeks may be selected. Common adverse effects include diarrhea, nausea, abdominal pain, vomiting, anorexia, constipation, mucositis, neutropenia, leucopenia, anemia, thrombocytopenia, asthenia, pain, fever, increased bilirubin, alopecia, dyspnea, cough, and dizziness.19
Saltz et al showed the combination of irinotecan, fluorouracil, and leucovorin to be active in the treatment of colorectal cancer.20 In this phase III study, patients with metastatic colorectal cancer were randomized to receive irinotecan with leucovorin and fluorouracil weekly for four weeks every six weeks, weekly irinotecan alone for four weeks every six weeks, or fluorouracil and leucovorin daily for five consecutive days every four weeks. Progression-free survival (PFS) in the three-drug arm was significantly longer than in the two-drug regimen, while the irinotecan-alone group had similar PFS to the two-drug arm. The objective response rate was also significantly greater in patients receiving the three-drug regimen versus the two-drug regimen or versus monotherapy with irinotecan. Finally, median survival of patients receiving triple-drug therapy was slightly longer as well compared with the other two treatment arms. Although irinotecan seemed to provide an advantage regarding clinical efficacy, adverse effects were observed at a higher rate and severity in patients who received irinotecan. Twenty-two percent of patients receiving the three-drug regimen and 31% of patients receiving irinotecan alone developed grade 3 or 4 diarrhea, compared with only 13.2% in patients receiving fluorouracil. Grade 3 or 4 nausea and vomiting was also higher in patients receiving irinotecan. In contrast, mucositis, neutropenia, and neutropenic complications were observed at a higher incidence in patients receiving the fluorouracil and leucovorin without irinotecan.20
Interestingly, in another comparative trial of irinotecan with fluorouracil plus leucovorin, the triple-drug combination was found to be no better than fluorouracil plus leucovorin.21 This trial was also conducted by Saltz et al and randomized patients with stage III colon cancer following surgical resection of the tumor. Patients received either leucovorin with fluorouracil or irinotecan followed by leucovorin and fluorouracil. Analyses revealed no statistical difference in overall three- or five-year survival, disease-free survival after three or five years, or recurrence-free survival after three or five years between the treatment groups. Patients in the three-drug regimen, however, were more likely to develop neutropenia and leucopenia at or exceeding grade 3 toxicity severity.21
Another common application of irinotecan with fluorouracil and leucovorin is the FOLFIRI regimen.22 In a noncomparative trial, the Gruppo Oncologico dell'Italia Meridionale evaluated the activity of irinotecan plus leucovorin followed by fluorouracil in patients with locally advanced and/or metastatic colorectal cancer. The entire three-drug regimen was given to patients every two weeks. Of the 264 assessable patients, 12 patients (4.5%) achieved a complete response and 86 patients (32.6%) achieved a partial response, while 106 patients (40.2%) had stable disease and 60 patients (22.7%) had progressive disease. The median duration of response was 10.5 months, with a median time to progression of seven months and median overall survival of 14 months.22
The results of these studies seem confounding due to the variability of results as well as the use of different doses. If irinotecan is to be selected for use in combination with fluorouracil and leucovorin, clinicians should consider the risk of adverse effects and other patient characteristics with this treatment regimen. Furthermore, the optimal dose of each of the three drugs also remains controversial.
Bevacizumab: Targeted therapies allow for a direct effect on the activities of cancer cells by interfering with direct actions of tumor growth. Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF).8 VEGF is a key regulatory protein that promotes the growth of endothelial cells. By targeting VEGF with monoclonal antibodies, neovascularization of tumors is inhibited, which suppresses tumor growth.23
Bevacizumab (Avastin) is approved for use as first- or second-line therapy in combination with fluorouracil-based chemotherapy for patients with metastatic colon cancer.24 Bevacizumab should be given every 14 days at 5 mg/kg when used in combination with 5-FU, leucovorin, and irinotecan and at 10 mg/kg when used with 5-FU, leucovorin, and oxaliplatin. The initial doses of bevacizumab are administered by intravenous infusion over 90 minutes. The second infusion can be given over 60 minutes if the first dose is well tolerated. Then, subsequent infusions can be given over just 30 minutes if successful tolerance of the second dose occurs. Common adverse effects seen after administration of bevacizumab in combination with fluorouracil-based regimens include diarrhea, leukopenia, neutropenia, pain, abdominal pain, headache, hypertension, vomiting, anorexia, constipation, stomatitis, dyspepsia, gastrointestinal hemorrhage, dizziness, upper respiratory infection, epistaxis, dyspnea, alopecia, and proteinuria. Increased caution is warranted following administration of bevacizumab due to the potential for gastrointestinal perforation, which may present as abdominal pain with constipation or vomiting. Wound healing can also be impaired; therefore, patients considering elective surgery should wait at least 20 days following the last dose of bevacizumab before undergoing surgical procedures.24
The effect of combining bevacizumab with fluorouracil and leucovorin has been compared with fluorouracil and leucovorin in a randomized trial.25 In this study conducted by Kabbinavar et al, patients with metastatic colorectal cancer received leucovorin followed by fluorouracil once weekly for six weeks of an eight-week cycle repeated until disease progression. Patients could receive fluorouracil with leucovorin alone, or in combination with low- or high-dose bevacizumab. Patients who received bevacizumab 10 mg/kg intravenously every two weeks until disease progression had a prolonged median survival compared with patients who received bevacizumab 5 mg/kg. Patients who received only fluorouracil and leucovorin had a median survival of 16.1 months.25
Future Directions
The development of new therapies for treatment of colon cancer, such as those that target epidermal growth factor receptor (EGFR) and VEGF, provide the opportunity to test alternative combination regimens that may offer multiple lines of treatment and improved outcomes. Additional commercially available agents such as panitumumab and cetuximab provide opportunities to investigate the application of these agents, which interfere with growth signals in tumors.
Panitumumab is a monoclonal antibody that also targets human EGFR and inhibits the binding of additional molecules preventing autophosphorylation and kinase activation.26 These actions result in cell growth inhibition, induction of apoptosis, and decreased production of inflammatory cytokines and growth factors. Panitumumab (Vectibix) is approved for use as monotherapy in the treatment of EGFR-expressing metastatic colorectal cancer in patients who have progressed after receiving fluoropyrimidine-, oxaliplatin-, and irinotecan-containing regimens. Panitumumab is given at a dose of 6 mg/kg intravenously over 60 minutes every 14 days. Doses greater than 1,000 mg should be infused over 90 minutes. Common adverse effects that have been observed with panitumumab use include skin rash, hypomagnesemia, paronychia, fatigue, abdominal pain, nausea, and diarrhea. Infusion reactions associated with panitumumab use may include allergic reactions or anaphylaxis with bronchospasm, fever, chills, and hypotension in about 1% of patients.26 Panitumumab has already been shown to offer refractory patients the potential for improvement in median progression-free survival over patients treated with best supportive care.27
Cetuximab (Erbitux) is a monoclonal antibody that targets EGFR.28 By inhibiting the binding of epidermal growth factor to its receptor, cetuximab prevents the phosphorylation and downstream activation of additional kinases, which impairs tumor cell growth and leads to apoptosis and decreased matrix metalloproteinase and vascular endothelial growth factor production.28
Erbitux is approved for use as monotherapy in patients with EGFR-expressing metastatic colorectal cancer after failure of both irinotecan- and oxalipatin-based regimens or in patients who are intolerant to irinotecan-based chemotherapy regimens.28 Erbitux may also be used in combination with irinotecan in patients with EGFR-expressing metastatic colorectal cancer. The dose of cetuximab is the same regardless of the decision to combine the drug with other agents. The initial dose is 400 mg/m2 intravenously over two hours, followed by weekly doses of 250 mg/m2 given over 60 minutes. Dosing should continue until disease progression or intolerance to therapy. Common adverse effects observed after cetuximab administration include fatigue, rash, pruritus, nail changes, headache, diarrhea, and infection. Caution should be taken during the peri-infusion period of cetuximab due to the risk of adverse infusion reactions that may include bronchospasm, stridor, hoarseness, hypotension, loss of consciousness, and/or cardiac arrest. Premedication with an H1 antagonist is recommended.28 Cetuximab used as monotherapy or in combination with irinotecan or oxaliplatin has been shown to produce objective responses in patients refractory to previous chemotherapy treatments.29-31
Agents on the horizon, such as vatalanib, that target VEGF-R2 intracellular tyrosine kinase as well as other kinases of VEGF receptors, platelet-derived growth factor, and basic fibroblast growth factor may offer clinical advantages over existing agents.32 The application of genomic techniques to determine gene expression in clinical samples of tumors from affected patients may further assist the selection of particular treatment options.33 The identification of biologic markers may allow for additional classification of patients into high- and low-risk categories, which would provide further evidence in treatment selection.32
Conclusion
Colon cancer is one of the most common malignancies affecting individuals in the United States today. Many chemotherapy agents have been used for decades in various combinations and dosages. Newer molecular targeting agents have offered additional promise due to a better understanding of tumor biology. Many studies have been undertaken to identify the most favorable dose, schedule, and combination of drugs currently available. The results from these studies, the application of genomic principles, and the development of new drugs will hopefully reduce the negative impact colon cancer has on society in the future. In conclusion, the drugs available today offer clinicians a multitude of options to assist in the management of colon cancer.
REFERENCES
1. American Cancer Society. Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007.
2. National Comprehensive Cancer Network. Colorectal Screening. Practice Guidelines in Oncology-v.1.2008.
3. Greene FL, Page DL, Fleming ID. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag; 2002.
4. Kasper DL. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill Inc; 2005.
5. Devita VT, Lawrence TS, Rosenberg TA. Devita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
6. National Comprehensive Cancer Network. Colon Cancer. Practice Guidelines in Oncology- v.1.2008.
7. Chung KY, Saltz LB. Adjuvant therapy of colon cancer: current status and future directions. Cancer J.
8. Brunton LL. Goodman's & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill Inc; 2006.
9. Petrelli N, Douglas HA, Herrera L, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. J Clin Oncol. 1989;7:1419-1426.
10. de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French Intergroup Study. J Clin Oncol. 1997;15:808-815.
11. Meta-Analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301-308.
12. Adrucil (fluorouracil) package insert. Irvine, CA: Sicor Pharmaceuticals Inc; 2005.
13. Leucovorin (leucovorin calcium) package insert. Irvine, CA: Sicor Pharmaceuticals Inc; 2004.
14. Xeloda (capecitabine) package insert. Nutley, NJ: Roche Laboratories Inc; 2006.
15. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment in stage III colon cancer. NEJM. 2005;352:2696-2704.
16. Scheithauer W, McKendrick J, Begbie S, et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a ramdomized phase III study. Ann Oncol. 2003;14:1735-1743.
17. Eloxatin (oxaliplatin) package insert. Bridgewater, NJ: Sanofi-Aventis US LLC; 2006.
18. Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. NEJM. 2004;350:2343-2351.
19. Camptosar (irinotecan) package insert. Japan: Yakult Honsha Co, LTD and Daiichi Pharmaceutical Co, LTD; 2007.
20. Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. NEJM. 2000;343:905-914.
21. Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456-3461.
22. Maiello E, Gebbia V, Paoletti G, et al. FOLFIRI regimen in advanced colorectal cancer: the experience of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol. 2005;16(suppl 4):iv56-iv60.
23. Börgstrom P, Gold DP, Hillan KJ, et al. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203-4214.
24. Avastin (bevacizumab) package insert. South San Francisco, CA: Genentech Inc; 2008.
25. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65.
26. Vectibix (panitumumab) package insert. Thousand Oaks, CA: Amgen Inc; 2007.
27. Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664.
28. Erbitux (cetuximab) package insert. Princeton, NJ: Bristol-Myers Squibb Co; 2007.
29. Saltz LB, Meropol NJ, Loehrer PJ, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201-1208.
30. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. NEJM. 2004;351:337-345.
31. Tabernero J, Van Cutsem E, Diaz-Rudio E, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225-5232.
32. O'Dwyer PJ. The present and future of angiogenesis-directed treatments of colorectal cancer. Oncologist. 2006;11:992-999.
33. Harkin DP. Genomics and the impact of new technologies on the management of colorectal cancer. Oncologist. 2006;11:988-991.
34. Wolmark N, Wieand K, Kuebler JP et al. A phase III trial comparing FULV to FULV + oxaliplatin in stage II and III carcinoma of the colon. Results of NSABP protocol C-07 [abstract LBA 3500]. J Clin Oncol. 2005;23(S16).
35. da Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment for advanced colorectal cancer. J Clin Oncol. 2000;18:2438-2447.
36. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized clinical trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30.
37. Schmoll HJ, Cartwright T, Tabernero J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1864 patients. J Clin Oncol. 2007;13:192-197. 2007;25:102-109.





