US Pharm. 2021;46(10):14.
According to the FDA’s Center for Drug Evaluation and Research (CDER), 410 novel drugs have been approved over the past decade, with an average of approximately 40 approvals granted per year. To expedite the review and availability of drugs that treat serious conditions and/or fill an unmet medical need, several approaches may be used by the CDER, including Fast Track, Breakthrough Therapy, Priority Review, and Accelerated Approval. In 2020, the CDER employed at least one expedited review process for 67.9% of novel-drug approvals (i.e., 36 of 53), with the highest number of novel drugs approved in any given year being 59 (in 2018). Subsequently, the number of novel-drug approvals dropped by 18.6% (to 48) in 2019 but then increased by 10.4% (to 53) in 2020.
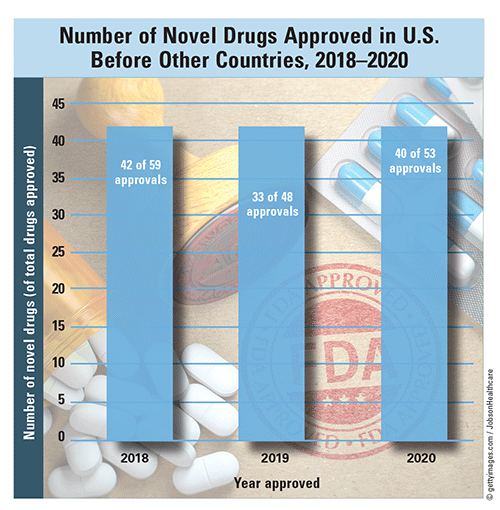
First-in-U.S. and First-in-Class Approvals: Between 2018 and 2020, 115 of 160 novel drugs were approved in the United States ahead of approval by any other country. U.S. approval rates of novel drugs in 2018, 2019, and 2020 were 71.2% (42 of 59 approvals), 68.8% (33 of 48 approvals), and 75.5% (40 of 53 approvals), respectively, of all drug approvals granted. In 2020, 39.6% of drug approvals were designated First-in-Class and included medications for infectious, neurologic, autoimmune, and endocrine diseases, among others. There were 23% more first-in-class approvals in 2020 than in 2018.
Accelerated, First-Cycle, and Fast-Track Approvals: In 2020, the CDER approved 12 of 53 novel drugs approved by the CDER had an Accelerated Approval designation, and 49 novel drugs (92.5%) were approved under the First-Cycle review process. Seventeen of the 53 novel drugs approved in 2020 (32.1%) were designated Fast-Track drugs. Approval rates for drugs designated Fast Track were 9.4% and 21.1% lower, respectively, than rates in 2019 (35.4%) and 2018 (40.7%).
Priority Review, Breakthrough Therapy, and Rare-Disease Approvals: Thirty of the 53 drugs approved in 2020 (56.6%) were designated Priority Review. The rate of Priority Review approval was lower than in 2019 (1.7%) and 2018 (16.3%). In 2020, 41.5% of drug approvals (22 of 53) were designated Breakthrough Therapy. This rate was an increase over the approval rates of 53.3% and 74.9% for 2019 and 2018, respectively. Between 2018 and 2020, 86 of the 160 novel drugs approved by the CDER were for the treatment of rare diseases. In 2018, 34 approvals for orphan drugs (a record high) were granted; 21 orphan drugs were approved in 2019; and in 2020, 58.5% of approvals (31 of 53) were for orphan drugs.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






