US Pharm. 2014;39(3)(Oncology suppl):3-6.
ABSTRACT: The specialty pharmacy market is growing, representing 25% of U.S. medication expenditures in 2011. The designation of specialty is vague, however, as both drugs and disease states can be classified as specialty. Rheumatoid arthritis (RA) is a disease state that is considered specialty. The pharmacotherapy of RA aims for disease remission through the principle of tight control, utilizing Disease Activity Scales (DAS) to assess a patient’s disease activity. Dispensing pharmacists are poised to provide the clinical monitoring and follow-up required in the specialty market by understanding the pharmacotherapy of RA, including DAS and the principle of tight control.
Specialty therapeutics were responsible for 25% of the United States’ medication expenditure in 2011, amounting to $80 billion.1 By 2018, six of the projected top 10 highest-grossing medications will be considered specialty therapeutics, with adalimumab (Humira) projected to be number one.2 Considered one of the most easily accessible healthcare providers, pharmacists are an integral part of the healthcare setting, including the specialty realm.
Although the definition of specialty pharmacy and therapeutics is vague, two criteria are usually found: high-cost treatment and the necessity for high-touch pharmacy services (i.e., benefits investigation, adherence monitoring, administration, and storage counseling) in order to manage care and ensure proper use of the therapeutics.3 The Centers for Medicaid and Medicare Services (CMS) defines specialty therapeutics as costing >$600 per month; additionally, the Academy of Managed Care Pharmacy (AMCP) has issued statements urging that high-dollar items are not the only designation for specialty.4 The other designations include, but are not limited to, difficulty in preparation, handling, or storage; complex patient management including monitoring and patient support systems; and Risk Evaluation and Mitigation Strategies (REMS).4
Not only are therapeutics considered specialty, disease states can also be considered specialty. For a disease to be classified as specialty, criteria include a low number of patients affected by the disease, high severity of disease if left untreated, and/or extensive monitoring for the disease or treatment as necessary. Disease states considered specialty include HIV, hepatitis C, Crohn’s disease, malignancies, and rheumatoid arthritis (RA), and the list continues to expand.
Rheumatoid Arthritis: Disease Overview
RA is an inflammatory autoimmune disease, which affects women at a higher rate than men.5 Patients with RA have an increased risk of premature mortality of approximately 50%, with a decrease in life expectancy by at least 3 years compared to the general population.6 In a recent study, Cole et al reported that 66% of employed patients with RA will experience job loss secondary to their disease state, further demonstrating RA’s disabling nature.7 In order to improve these statistics and patients’ quality of life, proper disease management is imperative.
In 2008, the American College of Rheumatology (ACR) published the first set of guidelines for the treatment of RA (Recommendations for the Use of Disease-Modifying Antirheumatic Drugs and Biologic Agents in the Treatment of Rheumatoid Arthritis), with an update in 2012.8,9 The guidelines address the goals of therapy for all patients with RA: early diagnosis, reduction of disease activity to low or remission, tight control through medical management, and prevention of further joint damage.
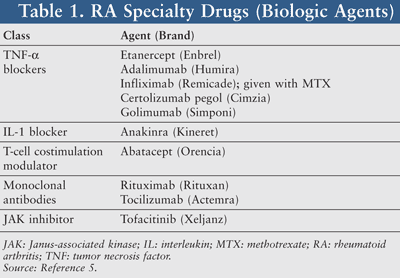
Following the goals of therapy, the guidelines discuss proper pharmacotherapy algorithms for patients with RA. The guidelines define disease-modifying antirheumatic drugs (DMARDs) as the foundation of treatment. The DMARDs can be classified as either biologic or nonbiologic, and are formulated as injectable or oral agents. A recent review article by Eaton et al provides a comprehensive medication chart for reference (see also TABLE 1 for a list of RA specialty drugs).5 Pharmacotherapy decisions are determined by disease activity, assessed by Disease Activity Scales (DAS), prognostic factors, and duration of disease. The DAS are tools to measure RA activity. These scales quantitate the disease activity into multiple categories ranging from remission to high disease activity, with remission the goal of therapy.
Disease Activity Scales
The 2012 ACR guidelines recommend using one of five DAS to evaluate RA disease activity.9 The five recommended DAS include the Patient Activity Scale (PAS/PAS II), Routine Assessment of Patient Index 3 (RAPID3), Clinical Disease Activity Index (CDAI), Disease Activity Score in 28 Joints (DAS28), and Simplified Disease Activity Index (SDAI).10-14 The guidelines do not direct a healthcare provider to a specific disease activity scale; however, each scale has benefits depending on the practice setting.9 A comparison of these scales is found in TABLE 2, highlighting which ones can be utilized by pharmacists.

The PAS/PAS II is a scale in which nonlaboratory data can be utilized to assess a patient’s disease activity. The scale includes questions regarding activities of daily living (e.g., walking on flat ground, getting on and off the toilet, moving heavy objects), pain scale, and overall assessment of daily life, using the Health Assessment Questionnaire (HAQ,). The PAS and PAS II are similar scales, differing in the HAQ used. The PAS II uses an abbreviated version of the HAQ, which allows for the scale to be completed at a quicker pace. After completing the questionnaire, the patient’s answers are scored and the disease activity is ranked as either in remission (≤0.25), minimal (≤3.7), moderate (<8.0), or severe (≥8.0).15
The RAPID3 is a scale similar to the PAS/PAS II. It has a three-component, self-administered scale including questions regarding activities of daily living (e.g., getting in and out of bed, lifting a full cup or glass to your mouth, walking 2 miles if you wish), a pain scale, and overall assessment of disease and its effect on daily living. The RAPID3 is an abbreviated scale of the Multi-Dimensional Health Assessment Questionnaire. Being an abbreviated scale, RAPID3 allows healthcare providers to quickly assess disease activity in patients with RA. The patient can self-administer the questionnaire and the provider can tabulate the answers and score the disease activity as near remission (0-1.0), low severity (1.1-2.0), moderate severity (2.1-4.0), and high severity (4.1-10.0).15
The CDAI is a disease activity scale that includes a swollen 28-joint count, a tender 28-joint count, a patient global assessment of disease activity, and provider global assessment of disease activity. The CDAI is a composite score of the four prior listed assessments with severity categories as follows: remission (0-2.8), low activity (2.9-10), moderate activity (10.1-22), and high activity (22.1-76.0).15
The DAS28 is a scale that includes a patient global assessment, as well as a provider evaluation of tender and swollen joints in 28 joints on both sides of the body. The DAS28 calculation also includes the erythrocyte sedimentation rate (ESR). If the ESR is not available, a C-reactive protein (CRP) level may be substituted. After completion, the scores can be evaluated as follows: remission (0-2.6), low disease activity (2.6-3.2), moderate disease activity (3.2-5.1), and high disease activity (>5.1).15
Like the CDAI, the SDAI includes a swollen 28-joint count, a tender 28-joint count, and a patient and provider global assessment of disease activity. However, the SDAI augments with a CRP level (mg/dL) in the calculation. After completion of the scale, the scores can be evaluated as follows: remission (0-3.3), low disease activity (3.4-11.0), moderate disease activity (11.1-26.0), and severe (26.1-86.0).15
Prognostic Factors
Therapeutic decisions are based on a patient’s disease activity but also the presence of poor prognostic factors. Poor prognosis for RA is defined in a patient with one of the following: active disease with swollen joints, evidence of radiographic erosions, elevated rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP), elevated ESR, and elevated CRP.8 However, the guidelines recommend certain prognostic factors, which should steer therapy, including functional limitations, extra-articular disease, positive RF and anti-CCP, and bony erosions on radiograph.8 Extra-articular disease includes pericarditis, pleuritis, rheumatoid lung disease, and vasculitis, all of which occur more frequently in patients who present with rheumatoid nodules.16 Presence of any or all of these symptoms is indicative of poor prognosis and is correlated with higher morbidity and mortality; therefore, more aggressive treatment is necessary to reach tight control and disease activity remission.
Tight Control
Once a patient’s disease activity and prognosis have been established, providers should begin treatment with DMARDs, following treatment algorithms recommended by the ACR guidelines.8,9 Treatment should be managed to a goal of tight control.8,9 Tight control of RA is defined as aggressive medical management with early initiation of DMARDs and quarterly visits with a rheumatologist. The ACR guidelines recommend tight control to achieve disease remission, which is supported by the Behandel-Strategieën voor Reumatoide Artritis (BeST trial), the Finnish Rheumatoid Arthritis Combination Therapy (FIN-RACo) trial, and the Tight Control of Rheumatoid Arthritis (TICORA) trial.17-19
The BeSt trial randomized 508 patients with early, active RA into four different therapeutic groups: sequential monotherapy (group 1), initial monotherapy with step-up combination as needed (group 2), combination therapy plus high-dose prednisone (group 3), or combination therapy including infliximab (group 4).17 Regimens were adjusted every 3 months based on DAS in 44 joints.17
Patients in groups 3 and 4 resulted in earlier functional improvement based on the Dutch version of the HAQ at 3 months; however, the difference became nonstatistically significant at 1 year.17 Additionally, a follow-up study reported that combination therapy benefit is limited to the initial year of treatment. At year 2, all groups had similar functional improvement, and 42% of patients in all groups achieved remission.20
The FIN-RACo trial evaluated outcomes of 199 patients randomized to either oral DMARD monotherapy (sulfasalazine with or without prednisolone) or combination therapy (sulfasalazine, methotrexate, hydroxychloroquine, prednisolone) for at least 2 years.18 Therapy was adjusted every 3 months in both study arms. The study was intention-to-treat with a goal to remain in remission.18 At 1 year, remission was achieved in 24 of 97 patients in the combination arm and 11 of 98 in the single-drug arm; however, the difference became statistically significant at 2 years.18
In 2004, a retrospective trial compared 5-year outcomes in terms of work productivity.21 The combination arm had a significantly lower cumulative duration of work disability per patient-year, with a median of 12.4 days versus 32.2 days.21 Additionally, a follow-up study at 11 years reported more patients on initial combination therapy achieved a reduction in disease activity, based on the modified Minimal Disease Activity measure (63% vs. 43%) and remission based on the ACR criteria (37% vs. 19%).22
Finally, the TICORA trial compared patients who were intensively managed and routinely managed.19 The intensively managed treatment arm included monthly rheumatologist appointments, monthly assessments of disease activity, glucocorticoid injections into swollen joints as needed, and pharmacotherapy adjustment every 3 months by predefined protocol.19 The routinely managed treatment arm included rheumatology visits every 3 months, no formal DAS utilized, and glucocorticoid injections or therapy modifications made at the discretion of the rheumatologist.19
All study outcomes favored the intensively managed group. At 18 months, the intensively managed group had a significant decrease in DAS (-3.5 vs. -1.9), higher proportion of good response based on European League Against Rheumatism criteria (82% vs. 44%), and better physical function assessed by the HAQ (-0.97 vs. -0.47).19
Succinctly, patients treated with tight control versus a less aggressive, nonguideline-based approach have better long-term outcomes, physical function, and disease activity and less utilization of sick time.23,24 A recurrent aspect of tight control is closely monitoring patients through the DAS. Pharmacists can be trained to monitor patients with RA and provide clinical data to clinicians by partnering together.
Conclusion and the Pharmacist’s Role
Patients with RA require intensive management and high-touch services from healthcare providers in order to reach remission. Pharmacists are in a position to contribute to the comprehensive care of patients with RA, especially with monitoring. Many of the DAS can be utilized at a dispensing level to monitor disease activity, thereby allowing pharmacists to provide a high-touch service. Dispensing pharmacists, community-based or otherwise, are in an ideal position to provide specialty services to patients with RA. As easily accessible healthcare providers with extensive therapeutic knowledge, pharmacists can become more involved with the management of RA through patient education, counseling, and monitoring of drug side effects.
REFERENCES
1. IMS Institute for Healthcare Informatics. The Use of Medicines in the United States: Review of 2011.
Parsippany, NJ: IMS Health Inc; April 2012.
www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/IHII_Medicines_in_U.S_Report_2011.pdf.
Accessed February 7, 2014.
2. Pembroke Consulting Analysis of World Preview 2013, Outlook to 2018.
Boston, MA: Evaluate-Pharma; July 2013.
www.evaluategroup.com/Public/Reports/Evaluate-World-Preview-2013-Outlook-to-2018.aspx.
Accessed February 7, 2014.
3. Hagerman J, Freed S, Rice G. Specialty pharmacy: a unique and growing industry. Pharmacy Today. July 1, 2013. www.pharmacist.com/specialty-pharmacy-unique-and-growing-industry. Accessed February 7, 2014.
4. Hernandez R. AMCP revises formulary document to reflect new recommendations for specialty pharma-ceuticals. Specialty Pharmacy Times.
January 29, 2013.
www.specialtypharmacytimes.com/news/AMCP-Revises-Formulary-Document-to-Reflect-New-Recommendations-for-Specialty-Pharmaceuticals.
Accessed February 7, 2014.
5. Eaton AT, Davis PN, Ndefo UA, Ebiogwu A. Update on pharmacotherapeutic options for rheumatoid arthritis. US Pharm. 2013;38(10):44-48. www.uspharmacist.com/content/d/feature/c/44278/. Accessed February 7, 2014.
6. Myasoedova E, Davis JM III, Crowson CS, Gabriel SE.
Epidemiology of rheumatoid arthritis: rheumatoid arthritis and
mortality. Curr Rheumatol Rep. 2010;12:379-385.
7. Cole JC, Li T, Lin P, et al. Treatment impact on
estimated medical expenditure and job loss likelihood in rheumatoid
arthritis: re-examining quality of life outcomes from a randomized
placebo-controlled clinical trial with abatacept. Rheumatology (Oxford). 2008;47:1044-1050.
8. Saag KG, Teng GG, Patkar NM, et al. American College of
Rheumatology 2008 recommendations for the use of nonbiologic and
biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762-784.
9. Singh JA, Furst DE, Bharat A, et al. 2012 update of the
2008 American College of Rheumatology recommendations for the use of
disease-modifying antirheumatic drugs and biologic agents in the
treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625.
10. Wolfe F, Michaud K, Pincus T. A composite disease
activity scale for clinical practice, observational studies, and
clinical trials: the Patient Activity Scale (PAS/PAS-II). J Rheumatol.
2005;32:2410-2415.
11. Pincus T, Yazici Y, Bergman M. A practical guide to
scoring a Multi-Dimensional Health Assessment Questionnaire (MDHAQ) and
Routine Assessment of Patient Index Data (RAPID) scores in 10-20 seconds
for use in standard clinical care, without rulers, calculators,
websites or computers. Best Pract Res Clin Rheumatol. 2007;21:755-787.
12. Aletaha D, Nell VP, Stamm T, et al. Acute phase
reactants add little to composite disease activity indices for
rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796-R806.
13. Fransen J, Stucki G, van Riel PL. Disease Activity
Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of
Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis
Disease Activity Index (RADAI). Arthritis Rheum. 2003;49(suppl):S214-S224.
14. Smolen JS, Breedveld FC, Schiff MH, et al. A
simplified disease activity index for rheumatoid arthritis for use in
clinical practice. Rheumatology (Oxford). 2003;42:244-257.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity. Arthritis Care Res. 2011;63(suppl 11):14-36.
16. Turesson C. Extra-articular rheumatoid arthritis. Curr Opin Rheumatol. 2013;25:360-366.
17. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart
CF, et al. Clinical and radiographic outcomes of four different
treatment strategies in patients with early rheumatoid arthritis (the
BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381-3390.
18. Möttönen T, Hannonen P, Leirisalo-Repo M, et al.
Comparison of combination therapy with single-drug therapy in early
rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353:1568-1573.
19. Grigor C, Capell H, Stirling A, et al. Effect of a
treatment strategy of tight control for rheumatoid arthritis (the TICORA
study): a single-blind randomised controlled trial. Lancet. 2004; 364:263-269.
20. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart
CF, et al. Comparison of treatment strategies in early rheumatoid
arthritis: a randomized trial. Ann Intern Med. 2007;146:406-415.
21. Puolakka K, Kautiainen H, Möttönen T, et al. Impact of
initial aggressive drug treatment with a combination of
disease-modifying antirheumatic drugs on the development of work
disability in early rheumatoid arthritis: a five-year randomized
follow-up trial. Arthritis Rheum. 2004;50:55-62.
22. Rantalaiho V, Korpela M, Hannonen P, et al. The good
initial response to therapy with a combination of traditional
disease-modifying antirheumatic drugs is sustained over time: the
eleven-year results of the Finnish rheumatoid arthritis combination
therapy trial. Arthritis Rheum. 2009;60:1222-1231.
23. Schur P, Moreland L. General principles of management of rheumatoid arthritis in adults. In: Basow DS, ed. UpToDate. Waltham, MA: UpToDate; 2013. www.uptodate.com. Accessed December 20, 2013.
24. Schur P, Cohen S. Treatment of rheumatoid arthritis resistant to initial DMARD therapy in adults. In: Basow DS, ed. UpToDate. Waltham, MA: UpToDate; 2013. www.uptodate.com. Accessed December 20, 2013.
To comment on this article, contact rdavidson@uspharmacist.com.






