US Pharm. 2019;44(8)(Specialty&Oncology suppl)3-7.
ABSTRACT: Systemic lupus erythematosus (SLE) is an autoimmune disease presenting with varying degrees of organ and system involvement. Treatment currently includes antimalarials, glucocorticoids, immunosuppressants, and biologics. Delayed diagnosis of SLE and decreased quality of life warrant an improvement in classification as well as in treatment. The American College of Rheumatology/European League Against Rheumatism classification criteria propose a point system to improve classification. Research in SLE continues with several drugs in various stages of development.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is challenging to diagnose and manage owing to its clinical heterogeneity and unknown cause. Unlike healthy individuals, patients with SLE have T and B cells that react to the patient’s own nucleic acids and binding proteins. These autoreactive T and B cells result in the development of autoantibodies leading to inflammation, tissue damage, and possible end-organ injury inflicted by the patient’s own immune system. It is speculated that genetic and environmental factors play a role in the etiology of SLE.1
Epidemiologic studies show a higher frequency of SLE in women—predominantly women of childbearing age—than men, with a ratio of 10:1. Incidence rates of SLE around the world are approximately 1 to 10 per 100,000 person/years, and prevalence rates range from 20 to 70 per 100,000 person/years. Persons of African, Asian, or Hispanic descent are at a higher risk of presenting with SLE than persons of European descent.1
The disease presents itself differently in every person, with varying degrees of organ and system involvement. Organ systems affected include the skin, joints, kidneys, lungs, central nervous system (CNS), and hematopoietic system. The initial presentation often includes fatigue, arthralgia, and the hallmark butterfly rash. However, these symptoms are not required for diagnosis. Other complications associated with the disease include vasculitis, alopecia, mouth ulcerations, nephritis, pericarditis, peripheral neuropathy, cognitive impairment, anxiety, and depression.2,3
Severe SLE can lead to inflammation of vital organs such as the brain and kidneys. Inflammation of the nervous system can lead to memory problems, confusion, strokes, and even seizures. The inflammation of the kidneys, referred to as lupus nephritis (LN), can damage the kidney and its ability to filter blood; 35% of SLE patients are found to have LN at SLE diagnosis and 50% to 60% develop LN within 10 years of SLE diagnosis. The severity ranges from class I to V, and in the event of renal failure, patients may require dialysis or kidney transplantation.4 Complications can be part of the initial presentation, but they may also develop over time as the disease progresses.
SLE is not to be confused with drug-induced lupus erythematosus (DILE). Drugs that commonly cause DILE include hydralazine, procainamide, isoniazid, minocycline, diltiazem, and tumor necrosis factor (TNF) inhibitors. Although presentation may be similar in arthralgia and fatigue, patients with DILE are less likely to present with organ damage or dermatologic manifestations. DILE flares have been observed months to years post initiation of the affiliated drug, and symptoms resolve within weeks of discontinuation.5
Owing to these variabilities in presentation, providers may take years to reach a diagnosis, leading to increased morbidities and a decline in patient-reported quality of life. A 2019 study used health-related quality of life (HRQoL) to assess SLE patients’ physical, mental, and social conditions. This study assessed 6,510 patients using the 36-item Short Form Health Survey (SF-36) with scores ranging from 0-100; higher scores imply a better health condition. SLE patients demonstrated a physical component score of 46.10 and a mental component score of 50.37. These patients scored lower physically and mentally than the general population and patients living with other chronic diseases, such as type 2 diabetes and hypertension. The lower SLE incidence rate may result in inadequate health services and. subsequently, lower HRQoL scores among this population.6 Although current treatments have improved the quality of life for patients, unmet needs remain. In addition to delayed diagnosis and lower QoL, problems such as insufficient disease control, comorbidities, and therapy toxicities warrant an increase in research to better understand the disease as well as develop novel products.7
Guidelines and Classifications
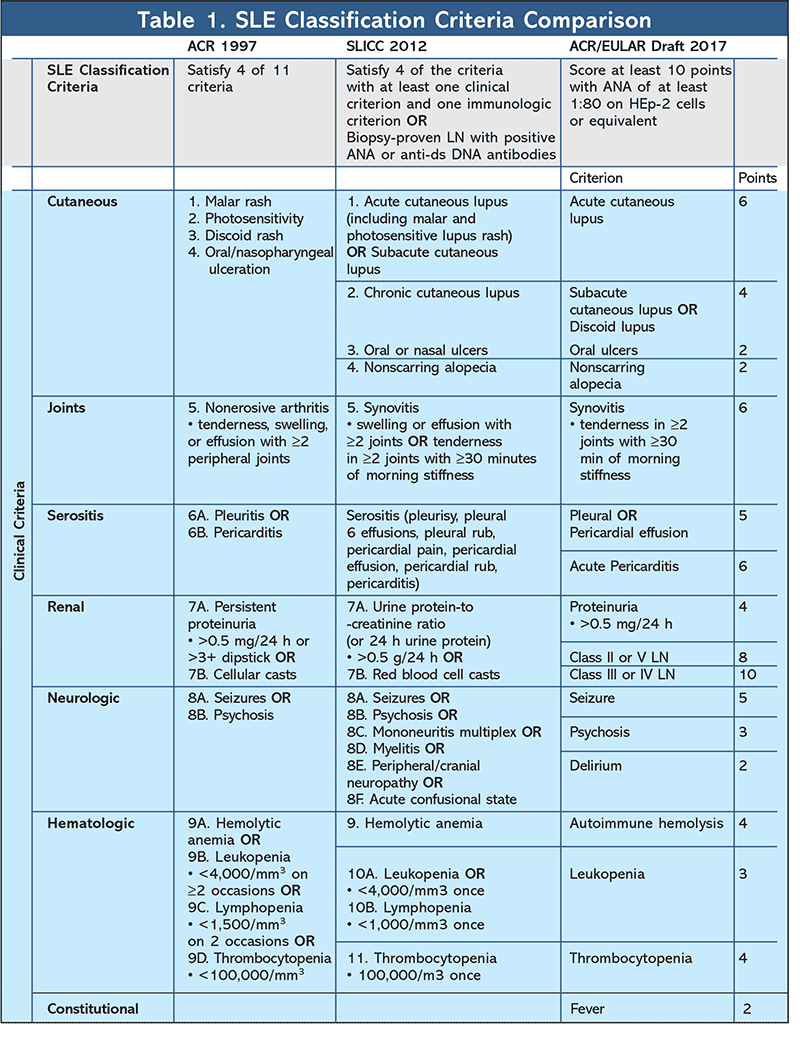
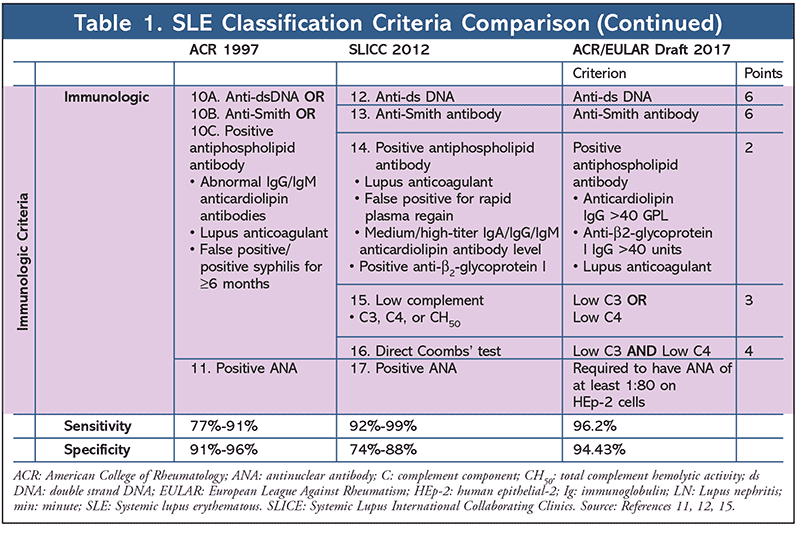
Previous SLE classifications include the American College of Rheumatology (ACR) criteria, revised in 1997, and the Systemic Lupus International Collaborating Clinics (SLICC) criteria published in 2012. Currently the ACR and European League Against Rheumatism (EULAR) are collaborating to develop new classifications for SLE. The new classifications were first presented at the 2017 ACR/Association of Rheumatology Health Professionals annual meeting in San Diego, California. The new classifications use a weighted approach as opposed to a minimum sign/symptom requirement, as did the previous ACR and SLICC criteria.
The ACR classification system consists of 11 criteria; four of these must be met for SLE classification.8,9 The SLICC signs and symptoms are classified as either clinical or immunologic. Patients must meet four of these criteria, with at least one clinical and one immunologic feature, or be diagnosed with biopsy-proven LN in addition to having a positive antinuclear antibody (ANA) or anti–double strand DNA antibodies to be classified for SLE.10
In the draft ACR/EULAR classification criteria, a point system is used for the 22 signs and symptoms. Each sign or symptom is assigned a value, and a patient must score at least 10 points and present with an ANA titer of at least 1:80 on human epithelial-2 (HEp-2) positive cells, or an equivalent, to meet SLE classification criteria. The benefits of this weighted classification include assessing patients who fall short of the minimum criteria in previous classification schemes as well as identifying whether patients with a higher score present with worse outcomes. These new classifications demonstrate 96.2% sensitivity and 94.43% specificity.11 Compared with the 77% to 91% sensitivity and 91% to 96% specificity of the 1997 ACR criteria, the new criteria have improved sensitivity and similar specificity. Compared with the 92% to 99% sensitivity and 74% to 88% specificity of the 2012 SLICC criteria, the new criteria have a similar sensitivity and improved specificity.12 The improved sensitivity and specificity, when compared with past criteria as a whole, help to more accurately classify SLE.13-15 See Table 1 for a comprehensive comparison of these criteria.
Treatment
There are many levels of severity and complications of SLE that require management. Treatment is dependent on presentation and options include antimalarials, glucocorticoids, immunosuppressants, and biologics. Nonsteroidal anti-inflammatory drugs (NSAIDs) may also be used to treat inflammation and pain.
Hydroxychloroquine (HCQ): HCQ is an antimalarial agent approved for SLE in 1957. It is used to treat cutaneous manifestations and arthralgia, as well as active renal disease, with concomitant immunosuppressive treatment.16 HCQ is often administered with systemic glucocorticoid agents as first-line treatment to improve long-term outcomes and overall survival.17 Patients using HCQ are at risk of retinal toxicity and are required to have routine ophthalmologic examinations. To minimize this risk, patients should be dosed with a maximum of 5 mg/kg/day according to actual body weight. If patients are unresponsive to HCQ, chloroquine may be considered; however, it carries a higher risk of retinopathy and should be closely monitored.
Glucocorticoids (GCs): GCs that are often used include methylprednisolone and prednisone. These agents are used to attain a rapid clinical response and decrease in inflammation in patients presenting with severe cases of SLE with active manifestations. Pulse therapy of 500-1,000 mg methylprednisolone daily for 3-5 days or 0.5-1 mg/kg prednisone taper may be used to bridge patients during initial or adjustment therapy. In addition to higher short-term doses of GC, some patients may require lower long-term doses to control their disease. Patients are often maintained on prednisone-equivalent doses of <7.5mg/day in efforts to avoid adverse effects, such as GC-induced osteoporosis.16
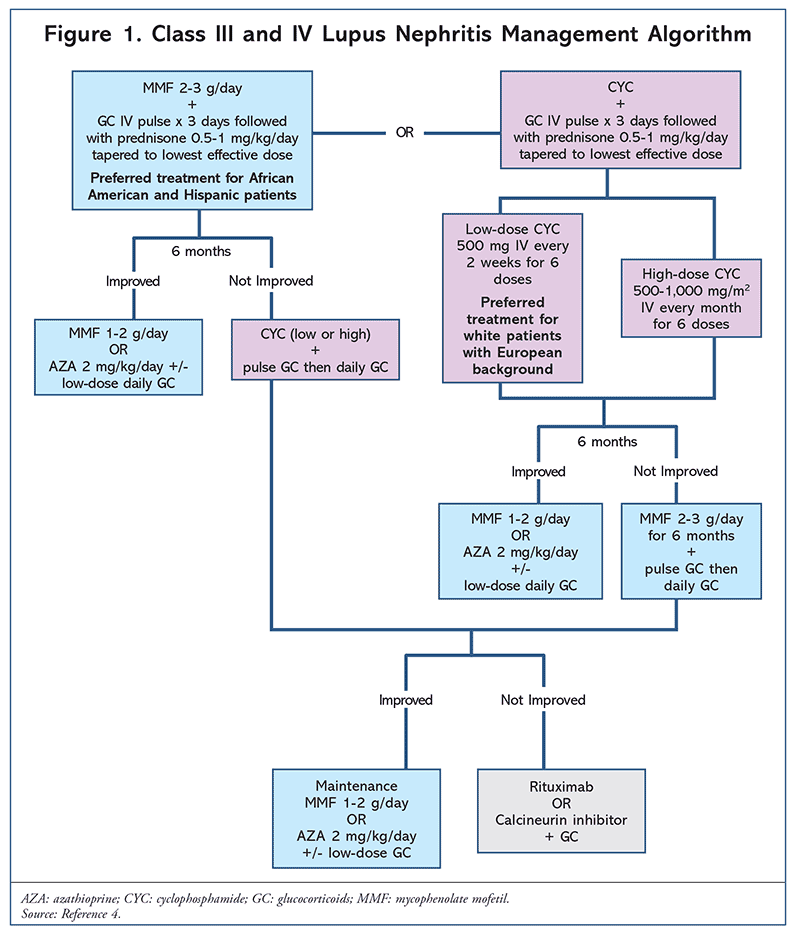
Immunosuppressants: Immunosuppressants include azathioprine (AZA), mycophenolate mofetil (MMF), and cyclophosphamide (CYC). These agents are indicated in patients with LN as well as in patients with other significant organ involvements. The treatment algorithm shown in Figure 1 is adapted from the ACR and describes class III and IV LN treatment in detail. Patients presenting with class III and IV LN are started on either MMF or low-/high-dose CYC, depending on the patient’s ethnicity. Initial treatment is maintained for 6 months before lowering the dosage or switching agents.4 AZA is a treatment option upon improvement following initial treatment; however, it is less effective than MMF in maintaining LN remission but is considered for pregnant patients as MMF and CYC are teratogenic.4,16
Biological Agents: Biological agents used in the treatment of SLE include rituximab and belimumab, both monoclonal antibodies. Rituximab targets B cells and is used to treat renal and CNS presentations of SLE. This agent is recognized as a second- or third-line agent for active disease. Belimumab targets the B-cell activating factor B-lymphocyte stimulator (BLyS).18 Belimumab is approved for use in active disease in conjunction with standard therapies including GCs, antimalarials, NSAIDs, MMF, and AZA. It is not, however, recommended for concomitant use with CYC or other biologics.16,19 It is the most recent FDA-approved drug for SLE; prior to belimumab’s approval in 2011, there had been no novel SLE-approved drugs for more than 50 years.20
Other Agents: Besides the agents listed above, there are other agents used off-label to treat SLE. These include disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, leflunomide, and calcineurin inhibitors (tacrolimus and cyclosporine). Other biologics, such as TNF inhibitors, abatacept, and tocilizumab, are also considered.16
Goals of Therapy: Therapeutic goals are individualized with the intention of achieving remission, preventing further organ damage, and improving the patient’s QoL. This may include monitoring disease activity (with instruments such as the Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index; Systemic Lupus Erythematosus Disease Activity Index 2000 [SLEDAI-2K], Systemic Lupus Activity Measure–Revised, or British Isles Lupus Assessment Group measure); preventing future flares; and aiming to decrease GC doses and concomitant drug toxicities.21
Pipeline Drugs
Current agents help with maintenance and symptomatic treatment of SLE. The unmet need and variability in SLE warrants new targeted therapies to further improve treatment beyond nonspecific immunosuppression. However, many agents have come and gone from pipelines without gaining approval. As of early 2019, rigerimod (Lupuzor), interferon-alpha (IFN) kinoid, atacicept, and obexelimab are in various stages of phase III investigations, and their approvals are highly anticipated. Targets for new drug development include major histocompatibility complex (MHC) class II receptors, IFN-alpha, BLyS/a proliferation–inducing ligand (APRIL), and CD19/FcyRIIb receptors. Additional potential novel products that are already approved in the United States but not currently indicated for SLE include ustekinumab and baricitinib, which target interleukin (IL)-12/IL-23 and Janus kinase (JAK)1/JAK2 inhibitors, respectively. Further investigation of these agents is warranted.
Rigerimod (Lupuzor): Rigerimod is a novel agent under development that modulates the activation of autoreactive T cells by targeting MHC class II receptors, thereby preventing the body’s production of autoantibodies and its resulting immune response. This upstream target of the immune cascade differs from existing products that target further downstream at the B-cell level. Phase III results have been reported, and rigerimod has received fast-track designation as well as special protocol assessment from the FDA.22 Rigerimod is part of the Managed Access Program, which allows early access to medication for patients prior to New Drug Application (NDA) approval; it is also part of an extension study with results anticipated in 2019.23 Rigerimod is advancing through the approval process.24
IFN-alpha Kinoid: IFN-alpha kinoid is a unique product in the pipeline. Unlike the other products, the IFN-alpha kinoid is injected IM into the participant as an immunization. The product is composed of a complex with inactivated IFN-alpha and a T-helper–stimulating carrier protein. The complex elicits antibody production against IFN-alpha, which is overproduced in patients with SLE and provokes inflammation. The antibody production prevents this inflammation. The phase IIb study showed statistical significance in reduction of steroid use as well as IFN-alpha at Week 36. The agent has been granted fast-track status by the FDA and is proceeding toward phase III investigation.25,26
Atacicept: Atacicept works by inhibiting the B-cell activation systems by targeting BLyS and APRIL.18 Weekly SC injections of either 75 mg, 150 mg, or placebo were administered to patients in its phase IIb study. Patients with high levels of disease activity at baseline were more likely to attain the endpoints of low disease activity, lupus low disease-activity state (LLDAS), and remission at 48 weeks taking atacicept 150 mg than those taking 75 mg or placebo. Flare reductions as well as an acceptable safety profile were also observed with atacicept treatment. The agent is progressing into phase III investigation.27-29
Obexelimab: Obexelimab (XmAb5871) targets CD19 and FcyRIIb receptors that inhibit B-cell activity when activated. In the phase II study, participants who achieved a reduction in lupus activity when withdrawn from immunosuppressive medications, excluding antimalarials, showed a positive trend in maintaining these improvements when given obexelimab. This agent is progressing into phase III investigation as well.30
Ustekinumab (Stelera): Ustekinumab is an IL-12 and IL-23 antagonist previously approved for plaque psoriasis, psoriatic arthritis, and Crohn’s disease. In a phase II study, ustekinumab met the primary endpoints for the study with 62% of patients on ustekinumab achieving an SLEDAI-2K responder index 4 (SRI-4) response at week 24 compared with the 33% of patients on placebo. This product is currently enrolled in, and recruiting for, a phase III investigation.31
Baricitinib (Olumiant): Baricitinib is a JAK1 and JAK2 inhibitor that blocks proinflammatory cytokines. It was approved in 2018 for its first indication, rheumatoid arthritis.32 The product’s phase II trial for SLE demonstrated statistically significant improvement in signs and symptoms of arthritis and rash, SRI-4 response, flare reduction, LLDAS, and tender-joint count in patients receiving baricitinib 4 mg daily. Baricitinib has proceeded to a phase III investigation and has been granted fast-track status by the FDA.33,34
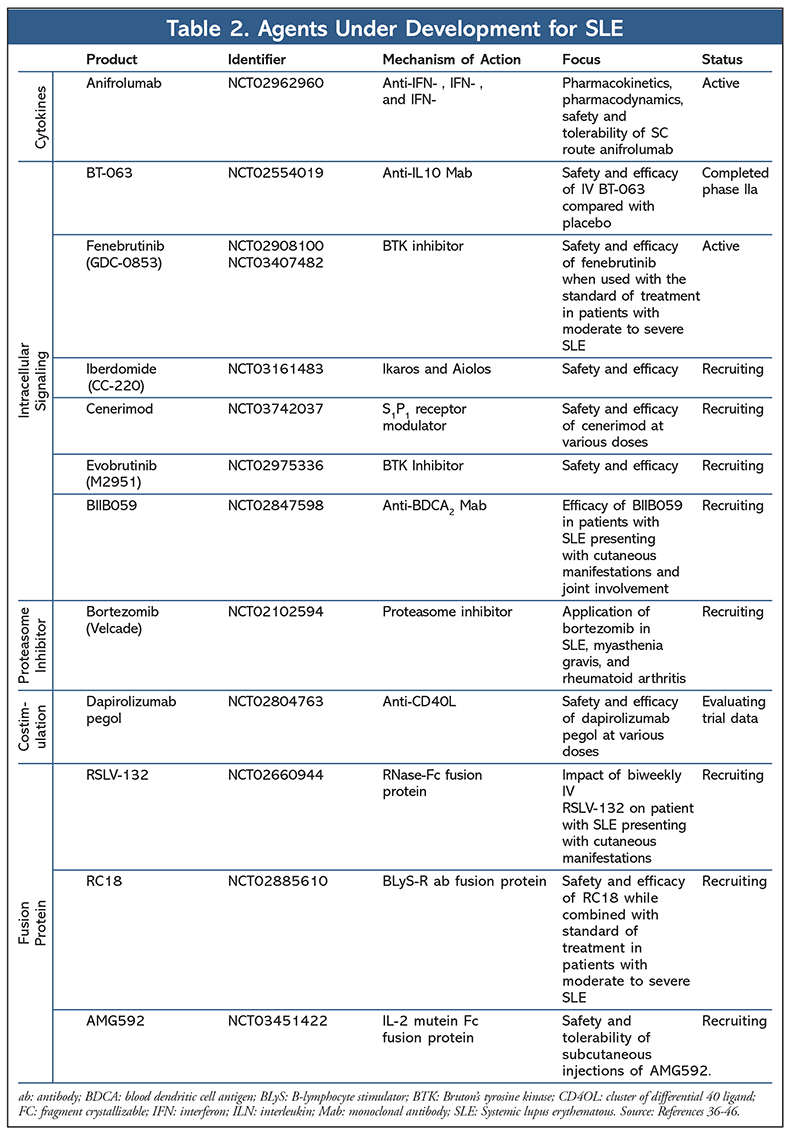
Pipeline: The pipeline for SLE has become robust and dynamic as compared with the last 50 years of drug development for this disease. Along with these agents, there are many ongoing phase II investigations as described in Table 2. These agents target cytokines, intracellular signaling, costimulation, proteasomes, and fusion proteins. Drugs can be, and often are, terminated at any stage of development. Although there are so many drugs currently in the works, the likelihood of approval throughout the phases is low. The Biotechnology Innovation Organization (BIO) initiated a study of the clinical drug-development success rate from 1,103 companies over a span of 9 years (2006-2015). Autoimmune drugs had an 11.1% probability of FDA approval from phase I, a 17% probability in phase II, a 53.5% probability in phase III, and an 86% probability after NDA or Biologic License Application submission.35 Although there are currently many SLE agents in phase II and III, data from this BIO study unfortunately predict a low probability of approval.
Conclusion
SLE treatment has made significant progress over the past decade; however, the management of SLE is complex, with a multitude of complications and various treatment options. Patients require a comprehensive plan for care and management of complications from both the disease and therapy. Pharmacists can be integrated into the care of SLE patients, especially in the areas of drug monitoring, adherence, and adverse-effect management as well as vaccination services and pain management. As pharmaceutical companies become increasingly interested in SLE, the investigational drugs in the pipeline will also continue to increase and, perhaps, beat the odds and lead to product approvals.
REFERENCES
1. Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257-268.
2. NIH U.S. National Library of Medicine. Systemic lupus erythematosus. https://ghr.nlm.nih.gov/condition/systemic-lupus-erythematosus#sourcesforpage. Accessed November 24, 2018.
3. CDC. Systemic lupus erythematosus. October 17, 2018. www.cdc.gov/lupus/facts/detailed.html. Accessed November 24, 2018.
4. Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012;64(6):797-808.
5. Mahdis S, Chin HH, Chauhan K. Drug-Induced Lupus Erythematosus. Treasure Island, FL: StatPearls Publishing; 2019. www.ncbi.nlm.nih.gov/books/NBK441889. Accessed June 28, 2019.
6. Gu M, Cheng Q, Wang X, et al. The impact of SLE on health-related quality of life assessed with SF-36: a systemic review and meta-analysis. Lupus. 2019;28(3):371-382.
7. Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res Ther. 2012;14(suppl 4):S4.
8. Pisetsky DS, Gilkeson G, St. Clair EW. Systemic lupus erythematosus. Med Clin. 1997;81(1):113-128.
9. Vu Lam N-CG, Maria V, Bieniek ML. Systemic lupus erythematosus: primary care approach to diagnosis and management. Am Fam Physician. 2016;94(4):284-294.
10. Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677-2686.
11. Zoler ML. New SLE classification criteria reset disease definition. MDedge. June 22, 2018. www.mdedge.com/rheumatologynews/article/168702/lupus-connective-tissue-diseases/new-sle-classification-criteria. Accessed October 19, 2018.
12. Freeman S. SLE classification criteria perform well in validation study. MDedge. June 22, 2018. www.mdedge.com/rheumatologynews/article/168694/lupus-connective-tissue-diseases/sle-classification-criteria-perform. Accessed June 22, 2018.
13. Petri M, Goldman D, Magder LS. Validation of proposed EULAR/ACR SLE classification criteria versus SLICC SLE classification criteria (abstract). Arthritis Rheumatol. 2018;70(suppl 10).
14. Adamichou C, Nikolopoulos D, Bortoluzzi A, et al. Increased sensitivity of the new (2017) and the 2012 SLICC as compared to the ACR 1997 classification criteria in early systemic lupus erythematosus (SLE): the 2017 and 2012 criteria may classify non-overlapping subgroups of patients (abstract). Arthritis Rheumatol. 2018;70(suppl 10).
15. Cunha JS, Gilek-Seibert K. Systemic lupus erythematosus: a review of the clinical approach to diagnosis and update on current targeted therapies. RI Med J. 2016;99(12):23-27.
16. Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford). 2017;56(suppl 1):i3-i13.
17. Muangchan C, van Vollenhoven RF, Bernatsky SR, et al. Treatment algorithms in systemic lupus erythematosus. Arthritis Care Res. 2015;67(9):1237-1245.
18. Cogollo E, Silva MA, Isenberg D. Profile of atacicept and its potential in the treatment of systemic lupus erythematosus. Drug Des Devel Ther. 2015;9:1331-1339.
19. Kim SS, Kirou KA, Erkan D. Belimumab in systemic lupus erythematosus: an update for clinicians. Ther Adv Chronic Dis. 2012;3(1):11-23.
20. Wallace DJ. The evolution of drug discovery in systemic lupus erythematosus. Nat Rev Rheumatol. 2015;11:616-620.
21. van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73:958-967.
22. Lupuzor. ImmuPharma PLC. www.immupharma.co.uk/folio/lupuzor. Accessed October 22, 2018.
23. Corporate Update (press release). London, England: ImmuPharma; September 7, 2018. www.immupharma.co.uk/wp-content/uploads/2018/09/IMM-September-2018-Corporate-Update.pdf. Accessed October 22, 2018.
24. Top line results of LupuzorTM pivotal phase III (press release). London, England: ImmuPharma PLC; April 17, 2018. www.immupharma.co.uk/top-line-results-lupuzor-pivotal-phase-iii-trial/. Accessed October 22, 2018.
25. Neovacs announces the results of its phase IIB study for IFN-alpha kinoid in the treatment of lupus which allows to proceed with the clinical development into phase III [press release]. Paris, France and Boston, MA: Neovacs; July 3, 2018. https://neovacs.fr/wp-content/uploads/2018/07/854553.pdf. Accessed October 22, 2018.
26. Neovacs announces its clinical advisory board meeting to design the phase III study for IFN-K in lupus (press release). Paris, France; Boston, MA: Neovacs; January 31, 2019. https://neovacs.fr/wp-content/uploads/2019/01/neovacs-_-cab-phase-iii_engversion_final-pdf. Accessed February 10, 2018.
27. Morand E, Merrill J, Isenberg DA, et al. Attainment of low disease activity and remission in SLE patients who started with high disease activity in the atacicept phase IIb address II study and its long-term extension (abstract). Arthritis Rheumatol. 2018;70(suppl 10).
28. Shen J, Papasouliotis O, Samy E, et al. Atacicept dose rationale for a phase 3 study in patients with high disease activity and auto-antibody positive SLE (abstract). Arthritis Rheumatol. 2018;70(suppl 10).
29. Mumal I. Atacicept, a B-cell targeting protein, reduced lupus flares in clinical trials. April 9, 2018. Lupus News Today. https://lupusnewstoday.com/2018/04/09/atacicept-reduced-lupus-flares-in-two-clinical-trials. Acessed January 20, 2019.
30. Xencor announces topline results from phase 2 study of XmAb®5871 in systemic lupus erythematosus and selection of late-breaking abstract for presentation at the 2018 ACR annual meeting (press release). Monrovia, CA: Xencor; October 5, 2018. https://investors.xencor.com/news-releases/news-release-details/xencor-announces-topline-results-phase-2-study-xmabr5871. October 5, 2018.
31. van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330-1339.
32. FDA approves Olumiant® (baricitinib) 2-mg tablets for the treatment of adults with moderately-to-severely active rheumatoid arthritis [press release]. Indianapolis, IN: Eli Lilly and Co; June 1, 2018. https://investor.lilly.com/news-releases/news-release-details/fda-approves-olumiantr-baricitinib-2-mg-tablets-treatment-adults. October 22, 2018.
33. Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222-231.
34. FDA grants fast track designation to the baricitinib development program for the treatment of systemic lupus erythematosus (SLE) (press release). Indianapolis, IN: Eli Lilly and Company; December 13, 2018. https://investor.lilly.com/news-releases/news-release-details/fda-grants-fast-track-designation-baricitinib-development.
35. Thomas DW, Burns J, Audette J, et al. Clinical development success rates 2006-2015. www.bio.org/sites/default/files/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf. Accessed February 1, 2019.
36. ClinicalTrials.gov. https://clinicaltrials.gov. Accessed April 11, 2019.
37. Kemp A. Update on TULIP 1 phase III trial for anifrolumab in systemic lupus erythematosus (press release). Cambridge, England: AstraZeneca; August, 31 2018. www.astrazeneca.com/media-centre/press-releases/2018/update-on-tulip-1-phase-iii-trial-for-anifrolumab-in-systemic-lupus-erythematosus-31082018.html. October 22, 2018.
38. Crawford JJ, Johnson AR, Misner DL, et al. Discovery of GDC-0853: a potent, selective, and noncovalent bruton’s tyrosine kinase inhibitor in early clinical development. J Med Chem. 2018;61(6):2227-2245.
39. UCB and Biogen announce topline results from a phase 2B study of dapirolizumab pegol in systemic lupus erythematosus [press release]. Brussels, Belgium and Cambridge, MA: Biogen; October 23, 2018. http://investors.biogen.com/news-releases/news-release-details/ucb-and-biogen-announce-topline-results-phase-2b-study. November 10, 2018.
40. Biotest AG: Phase IIa study demonstrates favorable safety and tolerability profile for Biotest’s monoclonal antibody BT-063 for treatment of systemic lupus erythematosus (SLE) (press release). Dreieich, Germany: Biotest AG; July 19, 2018.
www.biotest.com/de/en/investor_relations/news_and_publications/biotest_press_releases/press_detail.cfm?instance_ID=2768&cmfaction=xmldetail.xmldetail.detailview&showdetails=1709363. Accessed May 1, 2019.
41. Schafer PH, Ye Y, Wu L, et al. Cereblon modulator iberdomide induces degradation of the transcription factors Ikaros and Aiolos: immunomodulation in healthy volunteers and relevance to systemic lupus erythematosus. Ann Rheum Dis. 2018;77(10):1516-1523.
42. Piperdi B, Ling Y-H, Liebes L, et al. Bortezomib: understanding the mechanism of action. Mol Cancer Ther. 2011;10(11):2029.
43. Idorsia initiates a multiple-dose efficacy and safety study with cenerimod for the treatment of systemic lupus erythematosus (press release). Allschwil, Switzerland: Idorsia; January 7, 2019. www.idorsia.com/media/news-details?newsId=2230487. Accessed January 20, 2019.
44. Beguem Alankus Y, Grenningloh R, Haselmayer P, et al. Inhibition of Bruton’s tyrosine kinase (BTK) prevents inflammatory macrophage differentiation: a potential role in RA and SLE (abstract). Arthritis Rheumatol. 2018;70(suppl 10).
45. Furie R, Werth VP, Merola JF, et al. Biib059, a monoclonal antibody targeting bdca2, demonstrates evidence of proof of biological activity in subjects with cutaneous lupus. Lupus Sci Med. 2017;4(suppl 1):A35.
46. Burge DJ, Eisenman J, Byrnes-Blake K, et al. Safety, pharmacokinetics, and pharmacodynamics of RSLV-132, an RNase-Fc fusion protein in systemic lupus erythematosus: a randomized, double-blind, placebo-controlled study. Lupus. 2016;26(8):825-834.
To comment on this article, contact rdavidson@uspharmacist.com.