ABSTRACT: The risk for osteoporosis and fractures in women with breast cancer is increasing for several reasons. Chemotherapy regimens can induce premature ovarian failure and amenorrhea, leading to decreased bone density. New adjuvant treatments such as aromatase inhibitors reduce the risk of disease recurrence and improve long-term survival but increase bone loss and the risk of fractures. Bisphosphonates, along with a healthy lifestyle and adequate intake of calcium and vitamin D, are the treatments of choice in breast cancer patients with bone loss and accelerated bone turnover. Pharmacists are in a good position to counsel patients on ways to enhance compliance with bisphosphonates and to improve bone health.
Osteoporosis is an increasingly common problem in women diagnosed with breast cancer.1 Cytotoxic chemotherapy regimens can induce premature amenorrhea and ovarian failure, especially in older premenopausal women.2 The depletion of estrogen due to ovarian failure results in reduced bone mineral density (BMD) and an increased risk of fractures.2 In addition, although aromatase inhibitors (AIs) such as anastrozole, exemestane, and letrozole have been shown to reduce the risk of disease recurrence compared to tamoxifen in patients with hormone-receptor-positive (HR+) breast cancer, treatment with AIs increases bone turnover as a result of nearly complete estrogen depletion. This leads to loss of BMD and increased fracture rates.3 AI therapy results in more rapid bone loss than naturally occurring bone loss associated with menopause.3,4
Advances in adjuvant chemotherapies have prolonged disease-free survival and overall survival of patients with breast cancer; the 10-year probability of survival now exceeds 80%.5 Kanis et al revealed that the annual incidence of vertebral fracture was nearly five times higher in women diagnosed with breast cancer compared to controls.6 It is important to note that almost 80% of the women in the study received tamoxifen, and since AIs have replaced tamoxifen as the treatment of choice, the incidence of fracture is likely to be higher. Because many women diagnosed with breast cancer are likely to be long-term survivors, it is important to maintain bone health. Bisphosphonates are the treatment of choice to prevent bone loss and treat osteoporosis in patients with breast cancer.3,7
Diagnosis of Bone Loss in Patients With Breast Cancer
Bone strength is determined by a combination of bone density and bone quality.8 BMD is the primary tool used to assess bone strength. Currently, the method of choice for monitoring BMD is dual-energy x-ray absorptiometry (DXA) of the spine and hip. DXA is widely available, reliable, sensitive, and accurate and provides standardized reference measurements for normal subjects across a wide age range.3 The World Health Organization defines osteoporosis as a bone density that is 2.5 standard deviations (SD) below peak bone mass or mean BMD for young healthy women (expressed as the T-score).1,2 Osteopenia is defined as a T-score between -1 to -2.5 SD.1 Many factors contribute to bone health and the risk of fractures; therefore, a patient’s overall risk should be considered in addition to BMD (TABLE 1).

Guidelines for Managing Bone Loss in Breast Cancer
In 2003, the American Society of Clinical Oncology (ASCO) released evidence-based clinical practice guidelines for maintaining bone health specifically for women with breast cancer.1 Recommendations included an annual DXA assessment of the spine and hip in high-risk patients, as well as treatment with calcium (1,200 mg/day) and vitamin D (400 to 800 IU/day). ASCO recommends that patients with a T-score greater than -2.5 should be evaluated annually and those with a T-score of -2.5 or less receive a bisphosphonate in addition to calcium, vitamin D, and annual DXA scans.1
Guidelines recently released by an expert group in the United Kingdom modified the ASCO recommendations to reflect new information and take a more cautious approach for intervention.2 Reid et al advise that skeletal status should be assessed before starting treatment with an AI and to reflect the speed of treatment-induced bone loss; they suggest intervention when the T-score falls below -2 in postmenopausal women.2 In women with premature menopause receiving ovarian suppression plus an AI, intervention should begin if the T-score is -1, because bone loss in these women is very rapid (~16% over 3 years). In addition, women over 75 years old with one or more risk factor for osteoporotic fracture should be treated with a bisphosphonate regardless of BMD. These guidelines will initiate treatment in the early stages of bone loss, before the strength of the bone is compromised, and may improve the clinical benefit of therapy.2
Treatment for Osteoporosis in Breast Cancer
Many of the agents approved by the FDA for prevention and treatment of osteoporosis are not appropriate in women with breast cancer, including selective estrogen receptor modulators (e.g., tamoxifen), synthetic parathyroid hormone, and estrogen or estrogen plus progestin. Although no treatments are specifically approved for bone loss related to AI therapy, the bisphosphonates are the preferred intervention in bone loss associated with low BMD or evidence of rapid bone turnover seen in breast cancer therapy.1-3
Bisphosphonates are osteoclast inhibitors and act directly on the bone to inhibit bone resorption.4 A number of studies have evaluated the use of oral and intravenous bisphosphonates in AI-induced bone loss.9-14 In general, studies investigating oral agents including risedronate, alendronate, and ibandronate effectively maintained or improved bone mass in women with cancer-related bone loss and were well tolerated (TABLE 2).10-14
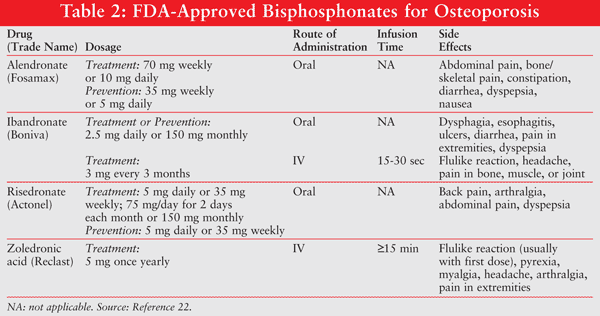
Although results are encouraging with treating bone loss in breast cancer with oral bisphosphonates, oral agents have low bioavailability as well as gastrointestinal (GI) intolerances that may interfere with optimal treatment. Moreover, the probability of long-term compliance is low; after one year of treatment with oral bisphosphonates, compliance may be as low as 50%.3 Until recently, IV bisphosphonates have not been used for the treatment of osteoporosis because of the inconvenience, as most physicians are not equipped to give IV infusions in their office. However, a single once-a-year dose of zoledronic acid was shown to be effective, and once or twice a year administration could improve compliance.15
Several studies have evaluated BMD-protective effects of IV zoledronic acid in adjuvant breast cancer treatment.16-18 In three large, parallel-designed studies (Zometa/Femara Adjuvant Synergy Trials: Z-Fast, ZO-Fast, and E-ZO-Fast), patients were randomized to start zoledronic acid 4 mg every six months either at initiation of the AI letrozole (immediate group) or at the time of BMD decline (T-score studies ≥2 or occurrence of fracture; delayed group).16-18 Although follow-up is not long enough at this point to assess fracture incidence, immediate treatment with zoledronic acid provided significant mean increases in BMD compared to delayed treatment. BMD increased by 2.0% and 1.4% in the lumbar spine and total hip, respectively, in the immediate treatment group. In contrast, among women in the delayed arm of the trial, BMD decreased by 2.6% and 2.1% in the lumbar spine and total hip, respectively (P <.0001 for each).16
Many cancers, including breast cancer, may metastasize to the skeleton, leading to severe bone pain, skeletal destruction, and pathologic fractures.19 Treatment with the IV bisphosphonates pamidronate, zoledronic acid, and ibandronate has also been shown to substantially relieve skeletal pain and reduce skeletal complications in patients with breast cancer bone metastases.19
Safety of Bisphosphonates
Currently, bisphosphonates are the most effective class of agents for treating osteoporosis and bone loss due to adjuvant chemotherapy. Although the frequency and severity of adverse events are generally mild, the route of administration influences the side-effect profile. Oral agents cause GI disturbances that can be managed by following administration guidelines from the manufacturer.
With IV administration, approximately 15% to 30% of patients experience an acute phase reaction characterized by transient fever and muscle and joint aches after the first infusion.19 Renal abnormalities have also been described when agents are given at high doses or as a rapid infusion.19,20 Recently, osteonecrosis of the jaw (ONJ) has been associated with the use of bisphosphonates.4,19,20 ONJ is an extremely painful condition that is commonly precipitated by a tooth extraction in patients treated with IV bisphosphonates (overall prevalence ~5%). Patients present with exposed bone in the mouth that fails to heal over a period of six to eight weeks.4 The diagnosis of ONJ is a clinical diagnosis by physical exam; biopsy of the affected bone can aggravate the situation. Management focuses on prevention, treatment of infection, and stopping bisphosphonate therapy.21

Counseling Points for Pharmacists
Guidelines released by ASCO stress that health care professionals, especially oncologists, should take an expanded role in routine and regular assessment of osteoporosis risk in women with breast cancer.1 It is important that pharmacists be aware of the risks of bone loss and osteoporosis associated with adjuvant therapy for breast cancer, as well as treatment strategies to improve bone health, minimize bone loss, and decrease the risk of fractures in patients diagnosed with breast cancer. Pharmacists should talk to patients with breast cancer about how to maintain bone strength with healthy lifestyle changes and to minimize the risk of fractures and other complications associated with adjuvant cancer treatments (TABLE 3).
REFERENCES
1. Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042-4057.
2. Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(suppl 1):S3-S18.
3. Coleman RE, Body JJ, Gralow JR, Lipton A. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treat Rev. 2008;34(suppl 1):S31-S42.
4. Hirbe A, Morgan EA, Uluckan O, Weilbaecher K. Skeletal complications of breast cancer therapies. Clin Cancer Res. 2006;12(suppl 20):6309s-6314s.
5. American Cancer Society. Breast Cancer Facts & Figures 2007-2008. Atlanta, GA: American Cancer Society, Inc.; 2007.
6. Kanis JA, McCloskey EV, Powles T, et al. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179-1181.
7. Coleman RE. Current and future status of adjuvant therapy for breast cancer. Cancer. 2003;97(suppl 3):880-886.
8. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17:1-45.
9. Brufsky AM. Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist. 2008;13:187-195.
10. Greenspan SL, Brufsky A, Lembersky BC, et al. Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol. 2008;26:2644-2652.
11. Delmas PD, Balena R, Confravreux E, et al. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol. 1997;15:955-962.
12. Eastell R, Van Poznak CH, Hannon RA, et al. The SABRE (Study of Anastrozole with the Bisphosphonate RisedronatE) study: 12-month analysis. J Bone Miner Res. 2007;22(suppl 1):S113.
13. Sawka AM, Ioannidis G, Papaioannou A, et al. Are oral bisphosphonates effective in improving lumbar bone mineral density in breast cancer survivors with osteopenia or osteoporosis? J Obstet Gynaecol Can. 2005;27:759-64.
14. Lester JE, Gutcher SA, Ellis SP, et al. Effect of monthly oral ibandronate on anastrozole-induced bone loss during adjuvant treatment for breast cancer: one-year results from the ARIBON study. J Clin Oncol. 2007;25(suppl):16s.
15. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
16. Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829-836.
17. De Boer R, Eidtmann H, Lluch A, et al. The ZO-FAST trial: zoledronic acid effectively inhibits aromatase inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: 24-month BMD results. Breast Cancer Res Treat. 2007;106(suppl 1):s36. Abstract 501.
18. Schenk N, Liombart A, Frassoladti A, et al. The E-ZO-FAST trial: zoledronic acid (ZA) effectively inhibits aromatase inhibitors associated bone loss (AIBL) in postmenopausal women (PMW) with early breast cancer (EBC) receiving adjuvant letrozole (LET). Eur J Cancer. 2007;5:186-187. Abstract 2008.
19. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
20. Coleman RE. Risks and benefits of bisphosphonates. Br J Cancer. 2008;98:1736-1740.
21. Reid IR. Osteonecrosis of the jaw: who gets it, and why? Bone. 2009;44:4-10.
22. Physician’s Desk Reference. 63rd ed. Montvale, NJ: Thomson; 2008.
To comment on this article, contact rdavidson@jobson.com.





