US Pharm. 2020;45(2)(Specialty &Oncology suppl):3-10.
ABSTRACT: Heparin-induced thrombocytopenia (HIT) is a potentially
life-threatening reaction to heparin that occurs in approximately 0.2% to 5% of patients and has an estimated mortality rate of 20%. The core management goal of HIT is to stop administration of heparin from all sources and initiate an alternative nonheparin anticoagulant. Current guidelines for the management of HIT recommend argatroban, danaparoid, bivalirudin, and fondaparinux to treat HIT. The current recommended agents require laboratory monitoring, parenteral administration, and a bridge to warfarin, rendering them labor-intensive and costly to healthcare systems. Direct oral anticoagulants have recently emerged as a potential option for the management of HIT.
Heparin-induced thrombocytopenia (HIT) is a potentially life-threatening reaction to heparin that occurs in approximately 0.2% to 5% of patients and has an estimated mortality rate of 20%. Higher rates of HIT have been noted in surgical patients receiving prolonged postoperative thromboprophylaxis with heparin products after orthopedic or cardiovascular surgery, but HIT can also occur regardless of dose, frequency, and route of administration.1-4 HIT can present in two types: type I and type II. Type I HIT is a mild, transient fall in platelet count that normally occurs within 48 hours of heparin exposure. The platelet count will return to normal with continued heparin administration. The platelet count typically does not drop below 100K/mcL. The mechanism of this transient drop is a nonimmune platelet aggregation caused directly by heparin’s effect on platelets. Type I HIT is not life-threatening and is not associated with thrombosis.
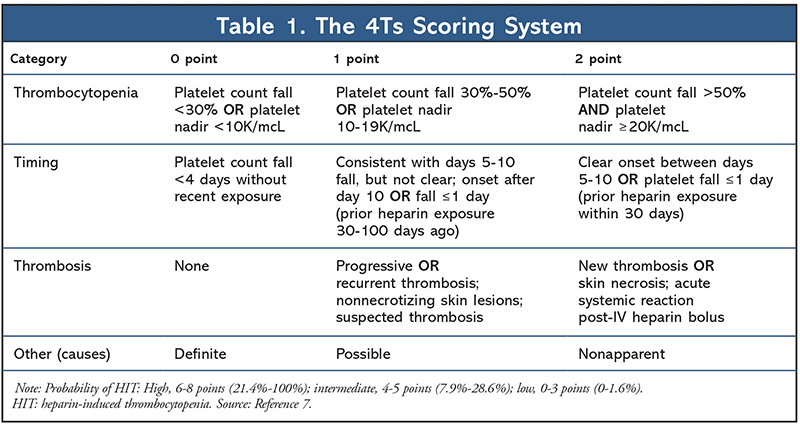
Type II HIT is a clinically significant, paradoxical, immune-mediated reaction, caused by the formation of antibodies that activate platelets after heparin administration. A key feature of type II HIT is a delayed onset of thrombocytopenia, usually within 5 to 10 days of heparin exposure.5 Heparin binds to platelet factor 4 (PF4), which forms an immunologic complex that leads to the formation of antibodies against the complex. After the antibody binds the heparin-PF4 complex, it proceeds to activate platelets, leading to the release of prothrombotic agents, which increases the risk of thrombus. The incidence of HIT-induced thrombus is estimated to be 40% to 50% and persists until platelet counts recover. The heparin-PF4-antibody-platelet complex is eventually cleared by macrophages, leading to thrombocytopenia.6 Timely identification and urgent treatment of HIT are essential in minimizing mortality and morbidity rates. The 4Ts score (TABLE 1) is widely used to determine an individual’s risk of having HIT.7 Patients with an intermediate-to-high risk warrant confirmatory testing that includes an anti-PF4/heparin enzyme-linked immunosorbent assay (ELISA) and a serotonin release assay (SRA). ELISA has a 100% negative predictive value for HIT; however, patients with a positive ELISA will need an SRA test to definitively diagnose HIT.8 SRA has a specificity of 95% and, in combination with ELISA, a sensitivity of 99%.9 This article will focus on the management of type II HIT and the updated literature available for direct oral anticoagulants (DOACs) in this setting.
Current Treatment Guidelines
The core management goal of HIT is to stop administration of heparin from all sources. Once heparin products have been discontinued, alternative anticoagulation methods should be evaluated (except warfarin monotherapy without bridging, owing to an increased risk of venous limb gangrene from its depletion of proteins C and S). Current guidelines for the management of HIT (set by the American College of Chest Physicians, the British Society of Hematology, and the American Society of Hematology [ASH]) recommend the use of argatroban, danaparoid, bivalirudin, and fondaparinux to treat HIT.7,10-12
Argatroban was approved in 2000 for prophylaxis or treatment of thrombosis in adult patients with HIT.13 A multicenter, prospective study on argatroban therapy for HIT showed improved outcomes in new thrombosis and deaths due to thrombosis.14 The treatment group received argatroban at an initial dose of 2 mcg/kg/min, which would be adjusted to achieve an activated partial thromboplastin time 1.5 to 3 times baseline values. The study used a historical control group who were treated according to the local practice of the hospital, which typically consisted of discontinuing heparin and initiating an oral vitamin K antagonist without a bridge. A composite outcome of all-cause death, amputation, or new thrombus was significantly reduced in patients treated with argatroban when compared with the control group (odds ratio, 0.61; 95% CI, 0.39-0.98; P = .04). The mean platelet count after 7 days was >150K/mcL.13 Additionally, in a trial conducted by the Cleveland Clinic, platelet counts had normalized after 6 to 7 days of treatment with argatroban.15
Bivalirudin was also approved in the United States in 2000, but received an indication for use as an anticoagulant in patients with or at risk of HIT undergoing percutaneous coronary intervention (PCI) in 2005.16 It is also commonly used off-label for patients with HIT or at risk of HIT with or without a planned PCI procedure. In a retrospective analysis of bivalirudin for HIT treatment, 461 patients were identified with a history of HIT, suspected HIT, and confirmed HIT who received bivalirudin. Of the 461 patients, 21 patients (4.6%) had experienced a new thrombus. Platelet counts had recovered to >150K/mcL within a median of 4 days in more than half of the patients with suspected or confirmed HIT (58.3%).17
Fondaparinux was approved in 2001, without an FDA indication for use in HIT. However, it is a frequently used agent (off-label) for the management of HIT.18 In a retrospective, national, multicenter observation registry study, fondaparinux was determined to be an effective agent to treat HIT. A total of 84 patients were identified who received fondaparinux for the management of suspected or confirmed HIT. None of the 84 patients had a new thrombus.19 In a separate retrospective study, it was shown that the median platelet recovery time (return to baseline) for fondaparinux was 9 days.20
Danaparoid is a low molecular weight heparinoid with fewer sulfur attachments than heparin; it is currently marketed outside of the U.S. It is used off-label for the treatment of HIT. In a randomized, controlled trial, 42 patients who were treated with danaparoid for 5 days followed by warfarin showed a trend toward reducing recurrent thrombosis without increasing the risk of major bleeding. Additionally, a retrospective cohort study reported that patients who received therapeutic doses of danaparoid compared with defibrinating snake venom had a significantly reduced risk of new thrombosis and major bleeding.10,21,22
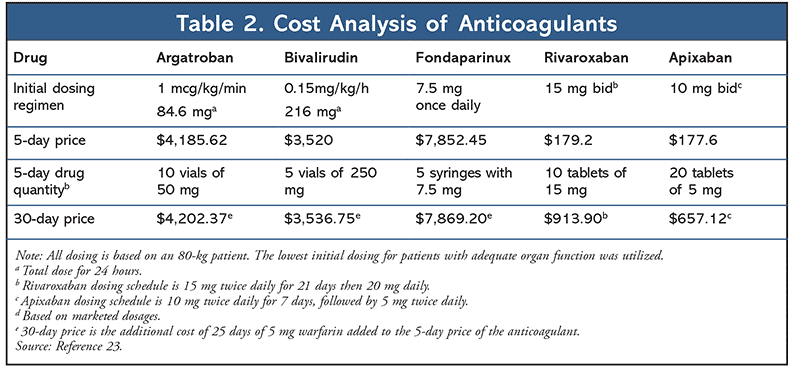
The basis of HIT treatment relies on nonheparin anticoagulants. The current recommended agents require laboratory monitoring, parenteral administration, and a bridge to warfarin, rendering them laborious and costly to healthcare systems (TABLE 2).3,23 In order to overcome these barriers, investigators have questioned the potential role of direct oral anticoagulants (DOACs) in the management of HIT. DOACs have not been observed to stimulate the formation of HIT antibodies, and their rapid onset of action without lowering the levels of proteins C and S suggest their viability in the management of HIT.21 In several in vitro tests, rivaroxaban and dabigatran had no effects on platelet activation, and did not interact with PF4 or anti-PF4/heparin antibodies.24 Additionally, rivaroxaban did not promote platelet aggregation at therapeutic levels.22 Promising in vitro studies led to the clinical application of DOACs in the management of HIT.
Current Literature on DOACS for HIT
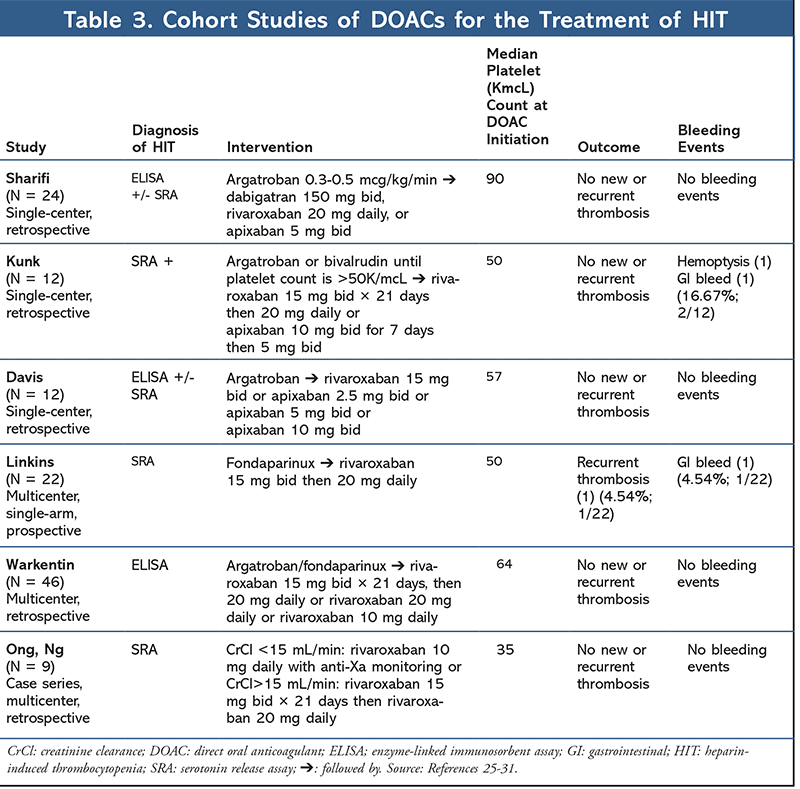
To date, there are six cohort studies (TABLE 3), 21 case reports, and one prospective trial, for a combined total of 104 patients treated with a DOAC for HIT. Three retrospective studies looked at multiple DOACs in the treatment of HIT. In a study conducted by Sharifi and colleagues, 22 patients were treated with rivaroxaban, dabigatran, or apixaban.25 Patients were initially treated with argatroban and then transitioned to a DOAC 2 hours after argatroban discontinuation. Median platelet count at the time of DOAC initiation was roughly 90K/mcL. Patients were followed for a median of 19 months. There were no new-onset cases of thromboembolism, limb loss, limb gangrene, or bleeding events reported.25
In a retrospective study conducted by Kunk and colleagues, 12 patients were treated with either rivaroxaban or apixaban for HIT.26 Treatment was initiated with either bivalirudin or argatroban and then transitioned to riva-roxaban or apixaban. All patients achieved platelet recovery within the first 6 days (range, 1-8 days), and there were no cases of new-onset thromboembolism or minor bleeds at a 6-month follow-up. However, two major bleeding events were reported that required discontinuation of therapy.26
A retrospective study conducted by Davis evaluated 12 patients treated with rivaroxaban or apixaban for HIT. Neither treatment led to new cases of thromboembolism or bleeding events. The median time to platelet recovery was 7 days (range, 3-14 days). Patients were only followed for approximately 1 month, with 11 patients continuing DOAC therapy after discharge. Postdischarge data on the 11 patients were unreported.27
Linkins and colleagues reported a multicenter, prospective cohort study for the use of rivaroxaban in HIT. Twelve patients were enrolled and were started on rivaroxaban 15 mg twice daily. Patients were deescalated to 20 mg daily after platelet counts recovered to >150K/mcL for those with HIT, or after 21 days for those with an HIT-related thrombosis. The primary outcome of a thromboembolic event had occurred in one patient. One patient was unable to achieve platelet recovery and was switched to fondaparinux. Based on the promising results, the authors expanded their study to include an additional 16 patients. Each patient received treatment for a minimum of a month, except for one who received therapy for 17 days. The median time to platelet recovery was 7 days (range, 4-29 days), with no reported thrombotic or major bleeding events at 30 days. With the addition of the 16 patients to the original 12, the rate of thrombosis was found to be 3.5%.28,29
Ong et al analyzed nine patients with end-stage renal disease (ESRD) and HIT-related thromboembolisms.30 Patients received rivaroxaban 15 mg twice daily then 20 mg daily after 21 days. If patients had a CrCl <15 mL/min, they received 10 mg daily. The mean platelet count at the time of rivaroxaban initiation was 35K/mcL. All patients recovered to platelet counts of >150K/mcL within a median of 8 days (range, 3-14 days). No recurrent thromboembolisms or bleeding events were reported.30,31
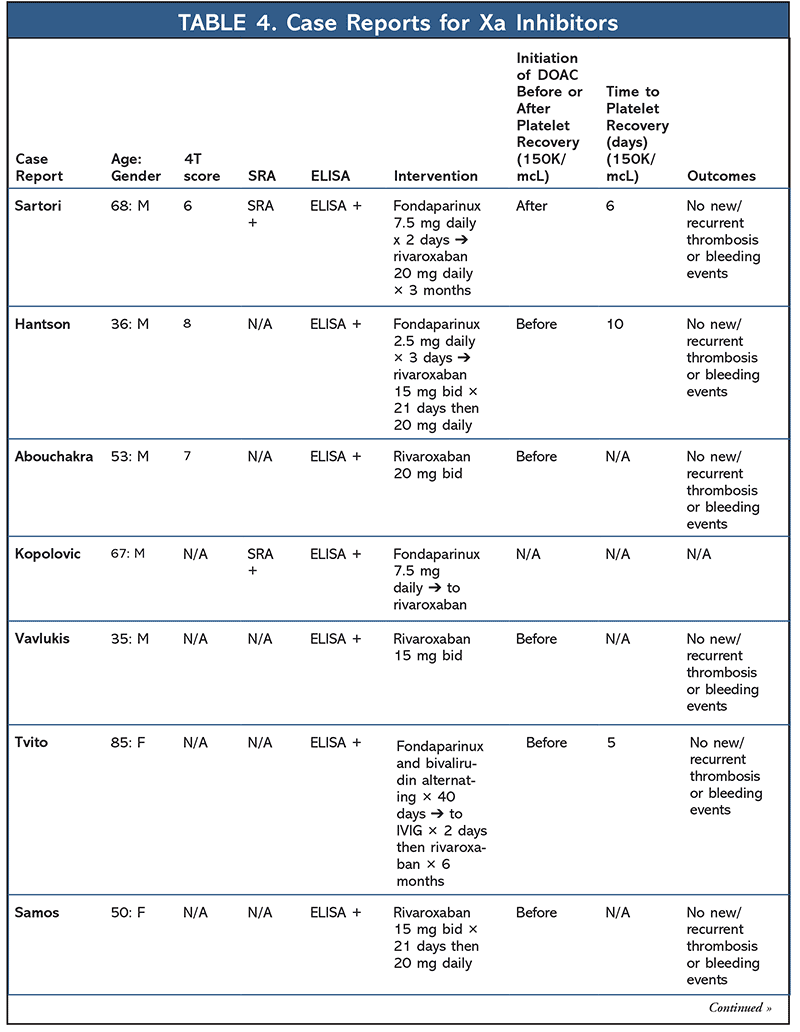
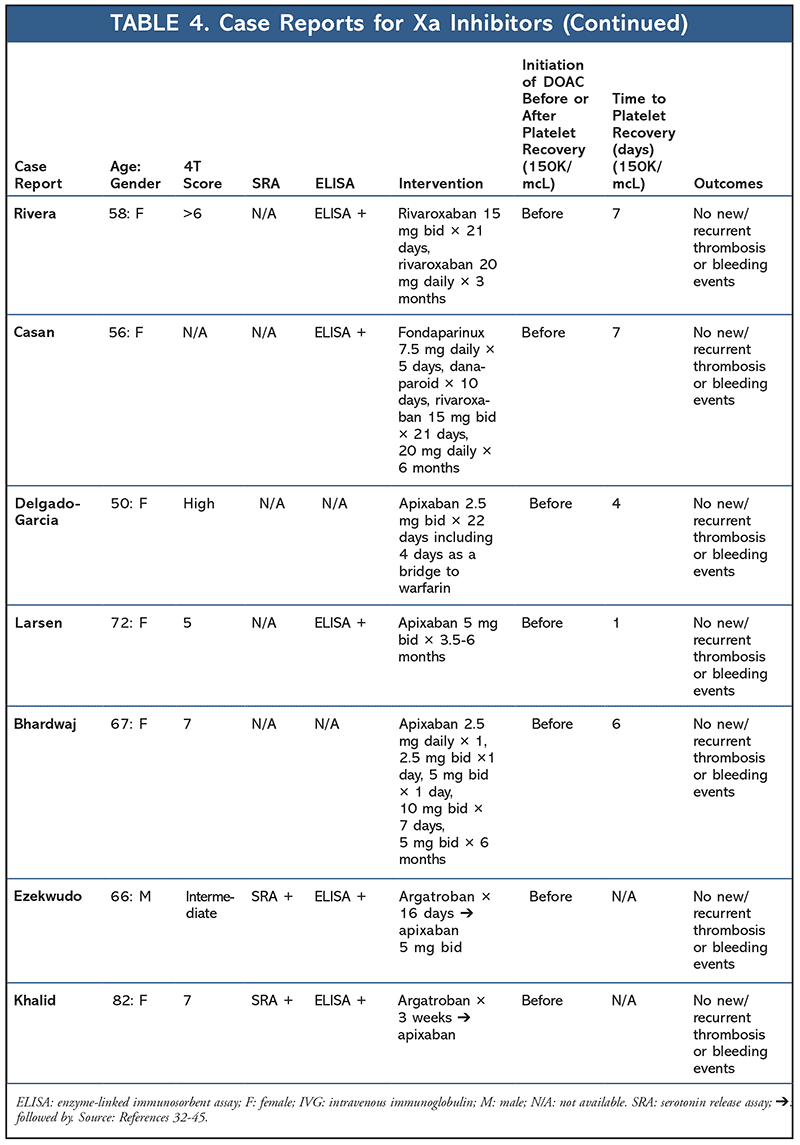
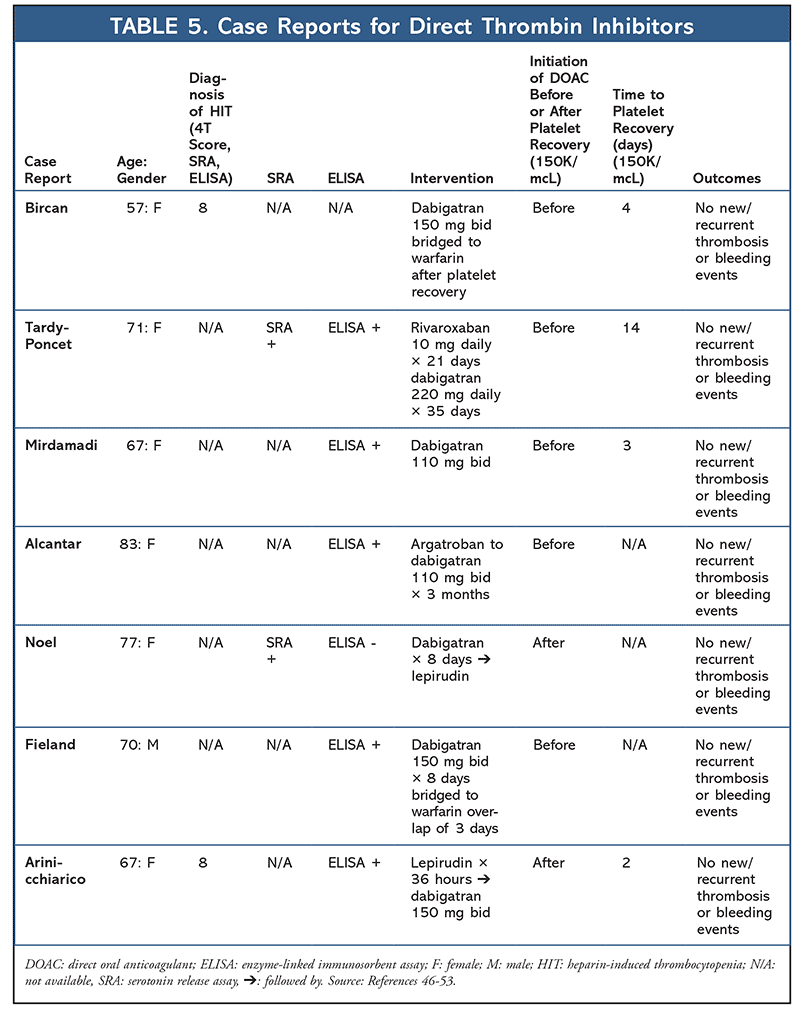
A total of 14 additional case reports have been published regarding the use of factor-Xa inhibitors (apixaban or rivaroxaban) in the setting of HIT (TABLE 4). Median time to platelet recovery was 6 days, with no reported thrombus or bleeding events. Seven case reports have also been published highlighting the use of dabigatran for HIT (TABLE 5). No new thromboembolisms or bleeding events were reported, with the median time to platelet recovery being 3.5 days (majority of cases did not report time to platelet recovery).32-52
Discussion
The current literature suggests DOACs have a potential role in the treatment of HIT. Out of the 104 patients who were identified, all but one patient achieved platelet recovery (150K/mcL) with a median time of 7 days. DOACs also prevented new or recurrent thromboembolisms in 98% (102) of cases, with only 3% (3) of patients experiencing bleeding events.
Given the growing evidence and promising results, the most recent guidelines released by ASH for the management of HIT in 2018 added DOACs as an option for the management of HIT. The preferred DOAC is rivaroxaban, with a recommended dosing regimen of 15 mg twice daily for 3 weeks followed by 20 mg once daily until the platelet count recovers to ≥150K/mcL.12 Apixaban can also be used at its venous thromboembolism (VTE)–treatment dosing.
Additionally, there is an ongoing Phase II clinical study analyzing the safety and efficacy of apixaban in the treatment of HIT (NCT03594045). Patients with HIT will receive apixaban at an initial dose of 10 mg orally twice a day for 7 days, followed by 5 mg twice a day for a total of 30 days.53 Patients will not be receiving an IV anticoagulant prior to apixaban initiation. The study’s primary outcome is the cumulative incidence of new symptomatic thromboembolism. This study will provide further evidence for the role of DOACs in HIT.
Despite the clinical efficacy of DOACs in the management of HIT, there are no studies directly comparing DOACs with the parenteral nonheparin anticoagulants. The ASH suggests choosing an agent based on availability, route of administration, half-life, and cost. DOACs have the potential to make a big impact on healthcare costs. Currently, parenteral nonheparin anticoagulant drugs are costly. TABLE 2 presents a general comparison of inpatient drug costs (over a 5- and 30-day period).21 DOACs offer a potentially more cost-effective therapy that allows for a quicker transition into outpatient management of HIT and ultimately reduces healthcare costs and resource use. Dabigatran was not included in the analysis as initial VTE treatment, but requires the addition of a parenteral anticoagulant for 5 to 10 days. Parenteral treatment is clearly more expensive, even without considering the additional costs of healthcare resources, equipment, and laboratory testing required with parenteral anticoagulants.
Conclusion
Parenteral nonheparin anticoagulants have been the cornerstone therapy for the management of HIT, but these agents are costly, require laboratory monitoring, and are given parenterally. DOACs, on the other hand, are oral agents that do not require routine laboratory monitoring. DOACs potentially provide a cost-effective and patient-friendly alternative for the management of HIT. Studies and case reports show promising results. Ideally, a head-to-head comparative trial with DOACs and parenteral anticoagulants would definitively establish the role of DOACs in HIT. However, the consistency of results in studies and reports, relatively low cost, and convenience warrant consideration of DOACs in the management of HIT.
Pharmacists can play a role in assisting with the management of patients with HIT by recommending the most cost-effective regimen for patients. Though DOACs have several advantages, these drugs do cost significantly more than warfarin. Pharmacists should consider the financial status of the patient and whether he or she would be able to afford DOACs as an outpatient. Additionally, if patients cannot reliably attend follow up appointments for international normalized ratio monitoring, DOACs may be a better option for them. If patients are unable to keep a consistent diet, warfarin may also not be ideal. DOACs are cleared renally and should be used cautiously in those with renal insufficiency. Recommending the right anticoagulant for a patient is essential for preventing repeat thrombotic events and reducing the risk of adverse events.
REFERENCES
1. Smythe MA, Koerber JM, Mattson JC. The incidence of recognized heparin-induced thrombocytopenia in a large, tertiary care teaching hospital. Chest. 2007;131(6):1644-1649.
2. Schmitt BP, Adelman B. Heparin-associated thrombocytopenia: a critical review and pooled analysis. Am J Med Sci. 1993;305(4):208-215.
3. Arepally G, Cines DB. Heparin-induced thrombocytopenia and thrombosis. Clin Rev Allergy Immunol. 1998;16(3):237-247.
4. Warkentin TE. Heparin-induced thrombocytopenia: a ten-year retrospective. Annu Rev Med. 1999;50:129-147.
5. Greinacher A. Clinical Practice. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261.
6. Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83(983):575-582.
7. Salter BS, Weiner MM, Trinh MA, et al. Heparin-induced thrombocytopenia: a comprehensive clinical review. J Am Coll Cardiol. 2016;67(21):2519-2532.
8. Nellen V, Sulzer I, Barizzi G, et al. Rapid exclusion or confirmation of heparin-induced thrombocytopenia: a single-center experience with 1,291 patients. Haematologica. 2012;97(1):89-97.
9. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572.
10. Linkins L, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2015;148(6):1529.
11. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159(5):528-540.
12. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392.
13. Argatroban package insert. Princeton, NJ: Sandoz Inc; 2000.
14. Lewis BE, Wallis DE, Leya F, et al. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163(15):1849-1856.
15. Bartholomew JR, Pietrangeli CE, Hursting MJ. Argatroban anticoagulation for heparin-induced thrombocytopenia in elderly patients. Drugs Aging. 2007;24(6):489-499.
16. Bivalirudin package insert. Parsippany, NJ: The Medicines Company; 2000.
17. Joseph L, Casanegra AI, Dhariwal M, et al. Bivalirudin for the treatment of patients with confirmed or suspected heparin-induced thrombocytopenia. J Thromb Haemost. 2014;12(7):1044-1053.
18. Schindewolf M, Steindl J, Beyer-Westendorf J, et al. Frequent off-label use of fondaparinux in patients with suspected acute heparin-induced thrombocytopenia (HIT)—findings from the GerHIT multi-centre registry study. Thromb Res. 2014;134(1):29-35.
19. Schindewolf M, Steindl J, Beyer-Westendorf J, et al. Use of fondaparinux off-label or approved anticoagulants for management of heparin-induced thrombocytopenia. J Am Coll Cardiol. 2017;70(21):2636-2648.
20. Snodgrass MN, Shields J, Rai H. Efficacy and safety of fondaparinux in patients with suspected heparin-induced thrombocytopenia. Clin Appl Thromb Hemost. 2016;22(8):712-717.
21. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130(9):1104-1113.
22. Walenga JM, Prechel M, Jeske W, et al. Rivaroxaban—an oral, direct factor Xa inhibitor has potential for the management of patients with heparin-induced thrombocytopenia. Br J Haematol. 2008;143:92-99.
23. Red Book Online. Ann Arbor, Michigan: Truven Health Analytics. https://redbook.solutions.aap.org/. Accessed November 15, 2019.
24. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119(5):1248-1255.
25. Sharifi M, Bay C, Vajo Z, et al. New oral anticoagulants in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015;135(4):607-609.
26. Kunk PR, Brown J, McShane M, et al. Direct oral anticoagulants in hypercoagulable states. J Thromb Thrombolysis. 2017;43(1):79-85.
27. Davis KA, Davis DO. Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur J Haematol. 2017;99(4):332-335.
28. Linkins LA, Warkentin TE, Pai M, et al. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost. 2016;14(6):1206-1210.
29. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130(9):1104-1113.
30. Ong SY, Chin YA, Than H, et al. Rivaroxaban for heparin-induced thrombocytopenia: adding to the evidence. Ann Hematol. 2017;96(3):525-527.
31. Ng HJ, Than H, Teo EC. First experiences with the use of rivaroxaban in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015;135(1):205-207.
32. Sartori M, Favaretto E, Cini M, et al. Rivaroxaban in the treatment of heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2015;40(3):392-394.
33. Hantson P, Lambert C, Hermans C. Rivaroxaban for arterial thrombosis related to heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2015;26(2):205-206.
34. Abouchakra L, Khabbaz Z, Abouassi S, et al. Rivaroxaban for treatment of heparin-induced thrombocytopenia after cardiac surgery: a case report. J Thorac Cardiovasc Surg. 2015;150(2):e19-e20.
35. Kopolovic I, Warkentin TE. Progressive thrombocytopenia after cardiac surgery in a 67-year-old man. CMAJ. 2014;186(12):929-933.
36. Vavlukis M, Kotlar I, Taravari H, et al. Can rivaroxaban be a drug of choice for treating heparin-induced thrombocytopenia in a patient with pulmonary thromboembolism? Anatol J Cardiol. 2017;18(1):77-79.
37. Tvito A, Bakchoul T, Rowe JM, et al. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am J Hematol. 2015;90(7):675-678.
38. Samoš M, Bolek T, Ivanková J, et al. Heparin-induced thrombocytopenia presenting with deep venous thrombosis and pulmonary embolism successfully treated with rivaroxaban: clinical case report and review of current experiences.J Cardiovasc Pharmacol. 2016;68(5):391-394.
39. Rivera OG, Corsi SO, Pavlovic JA, et al. Treatment of heparin-induced thrombocytopenia with rivaroxaban. Rev Med Chil. 2017;145(9):1213-1217.
40. Casan JM, Grigoriadis G, Chan N, et al. Rivaroxaban in treatment-refractory heparin-induced thrombocytopenia. BMJ Case Rep. August 12, 2016.
41. Delgado-Garcia G, Monreal-Robles R, Gallegos-Arguijo D, et al. Apixaban as therapeutic option in nephropathy patients with heparin-induced thrombocytopenia [in Spanish]. Gac Med Mex. 2015;151(6):798-801.
42. Larsen PB, Jørgensen M, Friis-Hansen L, et al. Apixaban used for the management of heparin-induced thrombocytopenia in a 72-year-old woman with lung cancer. Clin Case Rep. 2015;3(12):987-989.
43. Bhardwaj P, Petersen JR, Fornitz GG. The beneficial effect of apixaban in the treatment of heparin-induced thrombocytopenia. SM J Clin Med. 2018;4(1):1034.
44. Ezekwudo DE, Chacko R, Gbadamosi B, et al. Apixaban for treatment of confirmed heparin-induced thrombocytopenia: a case report and review of literature. Exp Hematol Oncol. 2017;6:21.
45. Khalid S, Daw H. The role of apixaban in the treatment of heparin-induced thrombocytopenia. Cureus. 2017;9(7):e1428.
46. Bircan HA, Alanoglu EG. Massive pulmonary embolism in a patient with heparin induced thrombocytopenia: successful treatment with dabigatran. Eurasian J Med. 2016;48(1):65-68.
47. Tardy-Poncet B, Piot M, Montmartin A, et al. Delayed-onset heparin-induced thrombocytopenia without thrombosis in a patient receiving postoperative thromboprophylaxis with rivaroxaban. Thromb Haemost. 2015;114(3):652-654.
48. Mirdamadi A. Dabigatran, a direct thrombin inhibitor, can be a life-saving treatment in heparin- induced thrombocytopenia. ARYA Atheroscler. 2013;9(1):112-114.
49. Alcantar JM, Chuang FL. Heparin-induced thrombocytopenia and the new oral anticoagulants. Proceedings of UCLA Healthcare. 2016;20.
50. Noel E, Abbas N, Skaradinskiy Y, et al. Heparin-induced thrombocytopenia in a patient with essential thrombocythemia: a case-based update. Case Rep Hematol. 2015;985253.
51. Fieland D, Taylor M. Dabigatran use in a postoperative coronary artery bypass surgery patient with nonvalvular atrial fibrillation and heparin-PF4 antibodies. Ann Pharmacother. 2012;46(1):e3.
52. Annicchiarico FJ, Alonso JL, Urbieta M, et al. Dabigatran as a therapeutic possibility in heparin-induced thrombocytopenia type II. An Sist Sanit Navar. 2012;35(3):521-524.
53. Massachusetts General Hospital. Efficacy and safety of apixaban in the treatment of heparin induced thrombocytopenia (HIT). https://clinicaltrials.gov/ct2/show/NCT03594045. NLM identifier: NCT03594045. Accessed July 1, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.