US Pharm. 2007;32(7)(Oncology suppl):5-10.
ABSTRACT: In the United States, it is
estimated that the frequency of venous thromboembolism (VTE) among cancer
patients is approximately 1 in 200. Aggressive prophylaxis and treatment of
thrombotic events should be initiated in this patient population, and research
shows that heparins are effective in the prevention and treatment of VTE--and
possibly survival--in oncology patients.
Oncology patients have a sevenfold overall
increased risk of venous thromboembolism (VTE) when compared to patients
without malignancy and are two to three times more likely to develop
postoperative thrombosis.1 Oncology patients who develop VTE are
also at increased risk for recurrent thrombosis compared to nononcology
patients (hazard ratio, 1.72).2 Venous thrombosis is the second
leading cause of death among oncology patients, after malignancy itself.2
In addition, thrombosis may be a presenting symptom of malignancy. An almost
10-fold increased incidence of cancer is seen in patients who have recurrent
idiopathic deep vein thrombosis (DVT) without known risk factors for
thrombosis.2
Etiology and Risk Factors
When monocytes and
macrophages interact with malignant cells, they release cytokines such as
tumor necrosis factor, interleukin-1, and interleukin-6, leading to sloughing
of endothelial cells and endothelial damage.3 These changes create
a thrombogenic vascular surface that increases the risk of VTE. The
interaction between macrophages and tumor cells also leads to activation of
platelets, factor XII, and factor X, initiating the clotting cascade and
leading to the formation of a thrombus.3
Cancer cells also release
substances that can increase thrombogenic risk. These substances, known as
cancer procoagulants, include cysteine proteases, tissue factor, and mucin
procoagulant.3,4 Tissue factor can also be released by monocytes
and macrophages in response to interaction with tumor cells.3
Cysteine proteases act to directly activate factor X, while tissue factor
directly activates factor VII.3 Mucin procoagulant, typically seen
in adenocarcinomas, works to activate prothrombin and causes nonenzymatic
activation of factor X.3,4
Venous stasis and endothelial
injury also place oncology patients at risk for VTE. In addition to
endothelial injury caused by the release of cytokines, factors such as
insertion of central venous catheters (CVCs) and chemotherapy administration
can damage the vascular lining.3.4 Venous stasis can be caused by
direct vessel compression by the tumor, CVCs, and patient immobility.3,4
Disordered blood flow and venous stasis may also be caused by angiogenesis,
which is induced by many tumors.4 Angiogenesis, the formation of
new blood vessels, produces vasculature that is abnormal in appearance.4
Flow through these vessels may vary in magnitude as well as direction.4
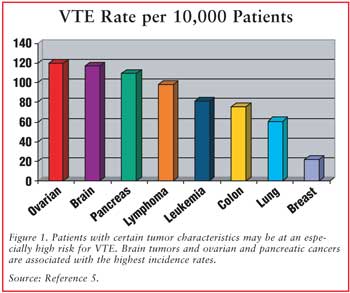
While all cancer patients are
at increased risk for thrombosis, those with certain tumor characteristics may
be at particularly high risk (see FIGURE 1). Primary brain tumors,
pancreatic cancer, and ovarian cancer are tumor types associated with the
highest frequency of VTE.2 In the Medicare Provider Analysis and
Review Record database, these malignancies were found to have a statistically
significant increased relative risk for VTE when compared with tumor types
associated with the lowest risk (i.e., head and neck, bladder, and esophageal
cancers).5 Despite a lower frequency of thrombotic events, tumor
types with the highest prevalence of VTE include breast, colorectal, and lung
cancers, due to the high incidence of these malignancies in the general
population.2 Patients with metastatic disease are also at increased
risk of VTE when compared with patients without metastasis.1
Agents used in the treatment
of malignancy can also be associated with increased risk for VTE. Chemotherapy
itself has been shown to increase VTE risk in several studies.6
Specifically, L-asparaginase can increase the risk for thrombosis due to
depletion of plasma asparagine, leading to decreased formation of proteins,
including the natural anticoagulants protein C and protein S.7
Exogenous estrogenic compounds, such as tamoxifen and diethylstilbestrol, used
to treat breast and prostate cancer, respectively, also place patients at
higher thrombotic risk.6 The estrogenic effects of oral
contraceptives used to prevent pregnancy during chemotherapy can act as an
additional risk factor in the oncology population.6 Other agents
associated with increased risk of VTE include erythropoietic growth factors
and thalidomide and lenalidomide, especially when combined with dexamethasone,
for the treatment of multiple myeloma.
Prophylaxis
The high risk of
thrombosis in the oncology population makes prophylaxis an important concern
in these patients. Factors that must be taken into consideration when
determining whether to use prophylaxis in a particular patient include recent
or current active bleeding, thrombocytopenia, severe platelet dysfunction,
recent major surgery at high risk for bleeding, need for spinal anesthesia or
lumbar punctures, recent central nervous system bleeding or intracranial
or spinal lesions at high risk for bleeding, and the patient's risk for falls.
6 All of these factors are considered relative contraindications to
anticoagulation and must be weighed in the decision of whether VTE prophylaxis
is warranted in an individual patient. The safety of anticoagulation must also
be considered in patients with underlying coagulopathy, including clotting
factor abnormalities and/or prolonged prothrombin time or activated
partial thromboplastin time.6 Patients who may be at particularly
high risk for VTE, requiring consideration of prophylaxis, include those
undergoing surgery and those who are hospitalized.
Cancer patients undergoing
surgery are twice as likely to develop postoperative DVT and three times as
likely to develop fatal pulmonary embolism (PE) as nononcology patients
undergoing similar procedures.8 A systematic review of randomized,
controlled trials in oncology patients revealed no difference in efficacy,
safety, or DVT location between low-dose unfractionated heparin (LDUH) and
low-molecular-weight heparin (LMWH) in surgical prophylaxis.9 A
statistically significant benefit was seen when higher doses of heparin
prophylaxis (defined as LMWH dose more than 3,400 U daily or LDUH 5,000 U
three times per day) were used, compared with lower doses.9 The
rate of DVT decreased from 12.7% to 5% when mechanical prophylaxis was added
to LMWH or LDUH prophylaxis.9
Hospitalized patients with a
diagnosis of active malignancy may be at increased risk of thrombosis due to a
lack of ambulation while hospitalized. Guidelines for VTE prophylaxis differ
in their recommendations.6,10 While guidelines from the National
Comprehensive Cancer Network (NCCN) suggest prophylaxis in all inpatients with
a diagnosis of active cancer,6 guidelines developed by the American
College of Chest Physicians (ACCP) discourage prophylactic anticoagulation in
hospitalized patients who are able to ambulate.10 The NCCN
recommends mechanical prophylaxis in all hospitalized cancer patients
concomitantly with pharmacological prophylaxis unless anticoagulant therapy is
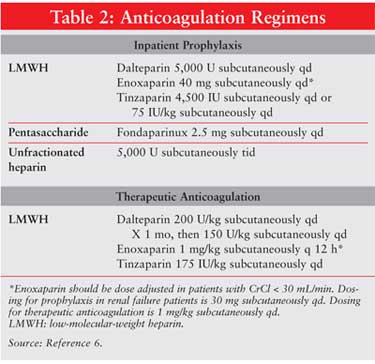
otherwise contraindicated.6 Prophylactic anticoagulation regimens
are shown in Table 1.
Patients with indwelling CVCs
are also at increased risk for thrombosis. While it was previously thought
that all patients with a CVC should receive VTE prophylaxis, randomized,
controlled trials have failed to show a benefit of such prophylaxis. A
double-blind, multicenter, randomized, controlled trial comparing enoxaparin
40 mg daily to placebo in 385 cancer patients scheduled to undergo CVC
insertion showed no statistically significant difference in the rate of DVT
between the two groups.11 Similarly, a randomized, multicenter
study comparing fixed-dose warfarin at 1 mg daily to placebo in cancer
patients after CVC insertion failed to show a benefit in the rates of
CVC-associated thrombosis.12 Secondary outcomes of
non-CVC–associated thrombosis, CVC life span, premature CVC removal, and CVC
lumen occlusions were not significantly different between the two groups.
12 Based on these data, routine prophylaxis of patients with CVCs is not
recommended by either the NCCN or ACCP.6,10
Treatment
In patients who
develop VTE, treatment and secondary prophylaxis are imperative due to the
increased risk for recurrent VTE seen in the oncology population. An ideal
treatment regimen would prevent recurrent DVT/PE without compromising patient
safety with regard to increased bleeding complications. This is of particular
concern in oncology patients who may have preexisting risk factors for
bleeding, such as thrombocytopenia or tumor types with a propensity to bleed
(e.g., renal cell carcinoma, melanoma). Thus, studies performed specifically
with an oncology patient population are most useful in characterizing
effective treatment regimens. Four such studies have been published comparing
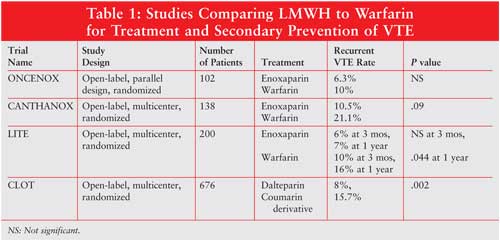
LMWH to warfarin for the treatment and secondary prevention of VTE (see
TABLE 1).13-16
Both the ONCENOX and CANTHANOX
trials compared enoxaparin to warfarin in cancer patients with VTE.13,14
Due to problems with accrual, both studies were closed early and included
only a small number of patients. The CANTHANOX trial was an open-label,
multicenter trial including 138 evaluable cancer patients with DVT and/or PE.
13 Patients were randomized to receive enoxaparin 1.5 mg/kg
subcutaneously once daily for three months or enoxaparin 1.5 mg/kg
subcutaneously once daily followed by oral warfarin, titrated to an
international normalized ratio (INR) between 2 and 3 for three months. Twenty
percent of the patients in the warfarin group experienced recurrent VTE or
major bleeding, compared with 10.5% of patients in the enoxaparin group. This
difference was not found to be statistically significant. A significant
difference was found between the two groups, however, in regard to the time to
event of primary outcome, with enoxaparin being superior (P = .04).
Patients randomized to the warfarin group were found to have a therapeutic INR
41% of the treatment time. Six patients in the warfarin group suffered death
related to bleeding, while no patients in the enoxaparin group had death
attributable to bleeding complications. No significant difference was found,
however, in major hemorrhage between the two groups.13
The ONCENOX, a pilot trial,
included 102 patients with active malignancy.14 Patients were
randomized to receive enoxaparin 1 mg/kg subcutaneously twice daily for five
days, followed by either enoxaparin 1.5 mg/kg once daily, enoxaparin 1 mg/kg
once daily, or warfarin, titrated to an INR between 2 and 3. Anticoagulation
was continued for a total of 180 days. There was no statistically significant
difference between the groups in the rates of symptomatic extension of the
diagnosed VTE or in recurrent VTE, nor in rates of bleeding.14
The LITE trial was a
randomized, multicenter, open-label trial involving 737 patients diagnosed
with VTE.15 A cancer subset of 200 patients was identified a
priori for separate analysis. These patients were randomized to receive
either tinzaparin 175 International Factor Xa Inhibitory Units per kilogram
subcutaneously once daily or unfractionated heparin followed by oral warfarin,
titrated to an INR between 2 and 3. Study drug was discontinued at 12 weeks
unless it was determined by the patient's primary care physician that further
oral anticoagulation was indicated. While the rates of recurrent VTE at the
end of the study period were not significantly different between the two
groups, the tinzaparin group had a significantly decreased rate of recurrent
VTE at one year (7% vs. 16%; P = .044). Bleeding complications were
similar between the two groups.15
Finally, the CLOT trial was an
open-label, multicenter study in 676 patients with active malignancy.16
Treatment randomization involved either dalteparin 200 U/kg subcutaneously
once daily for five to seven days and a coumarin derivative for six months or
dalteparin alone at a dose of 200 U/kg once daily for one month, followed by
150 U/kg once daily for five months. Follow-up at the end of the six-month
study period revealed that 27 of the 336 patients in the dalteparin group had
recurrent VTE, compared with 53 of the 336 patients in the oral anticoagulant
group (hazard ratio, 0.48; P = .002). The rates of major bleeding and
death at six months were not significantly different between the two groups.
Patients on oral anticoagulation were found to have INRs in the therapeutic
range 46% of the study duratin, with subtherapeutic INRs 30% and
supratherapeutic INRs 24%.16
Based on this data, LMWHs have
proven efficacy and safety in the prevention of recurrent VTE in the oncology
population. Agents with data supporting their superiority over warfarin
include dalteparin and tinzaparin.10,15,16 Dosages for LMWH in the
treatment of VTE are shown in Table 2.
LMWHs and Survival Benefit
Interest has been
generated in the role of LMWHs beyond the treatment and prevention of VTE.
While efficacy in the treatment setting has been established, it is thought
that LMWH may also play a role in tumor modulatory processes and thus may
increase survival in oncology patients. This idea is based on the theoretical
role of tissue factor beyond coagulation. Activation of tissue factor leads to
the upregulation of vascular endothelial growth factor, and downregulation of
thrombospondin-1, an antiangiogenic agent, resulting in an increase in
angiogenesis.1 LMWHs increase the release of tissue factor pathway
inhibitor, leading to an inhibition in tissue factor expression and,
theoretically, a decrease in angiogenic tumor activity.1,17 In
vitro experiments and experiments in animal models have shown that heparins
can interfere with angiogenesis as well as tumor invasion and adhesion of
cancer cells to the vascular endothelium, possibly having a role in the
prevention of metastasis--but not actual tumor growth. In vivo
studies in humans have also suggested a survival benefit of LMWH.18-20
A posthoc analysis of the CLOT
trial survival data at one year showed no difference in mortality between the
LMWH group and the oral anticoagulation group in the subset of patients with
metastatic disease.18However, in the group of patients without
metastasis, a survival advantage was seen in the dalteparin group at 12-month
follow-up (20% probability of death vs. 36% probability of death; P =
.03). It is thought that the lack of benefit seen in patients without
metastatic disease may be due to the effect of LMWH on modulation of
metastasis but lack of effect on tumor growth.18
A second trial, the FAMOUS
trial, examined the impact of LMWH on survival in cancer patients without
underlying VTE.19 This double-blind, placebo-controlled,
multicenter trial included 374 patients with solid malignancies and randomized
patients to receive either dalteparin 5,000 U subcutaneously once daily or
placebo for one year. Patients entering this study had to have a life
expectancy of at least three months from enrollment. No difference in survival
was seen in the study population as a whole at one, two, or three years. A
subgroup of patients, not defined a priori, was further analyzed for
survival benefit. This subgroup included patients with a better prognosis,
defined as those patients who were alive 17 months after randomization. Of
these patients, a significant survival advantage was seen at both two and
three years in the dalteparin group. There was no significant difference
between the two groups in regards to bleeding complications.19
The MALT trial was another
study conducted in oncology patients without underlying thrombosis.20
This study included 302 patients with metastatic or locally advanced solid
tumors. Patients were randomized to receive either nadroparin 9,500 IU
subcutaneously once daily or placebo for six weeks. Median survival was
increased from 6.6 months in the placebo group to eight months in the
nadroparin group (P = .021). The median survival advantage in the
nadroparin group was even more profound in a subset of patients with a life
expectancy greater than six months at randomization (15.4 vs. 9.4 months; P
= .01). The rate of clinically relevant bleeding was significantly increased
in the nadroparin group compared with placebo. Rates of major bleeding,
however, were similar between the two groups.20
Conclusion
Oncology patients
are at high risk for VTE. Aggressive prophylaxis and treatment of thrombotic
events should be initiated in this patient population. LMWHs have proven
efficacy and safety in cancer patients and are preferred over warfarin for the
prevention and treatment of VTE. Additional clinical trials are needed to
further define the role of LMWHs on survival results in oncology patients both
with and without underlying thrombosis.
References
1. Burris HA.
Low-molecular-weight heparins in the treatment of cancer-associated
thrombosis: a new standard of care? Semin Oncol. 2006;33:S3-S16.
2. Pruemer J.
Prevalence, causes, and impact of cancer-associated thrombosis. Am J Health
Syst Pharm. 2005;62:S4-S6.
3. Bick RL.
Cancer-associated thrombosis. N Eng J Med. 2003;349:109-111.
4. Letai A, Kuter DJ.
Cancer, coagulation, and anticoagulation. The Oncologist.
1999;4:443-449.
5. Levitan N, Dowlati
A, et al. Rates of initial and recurrent thromboembolic disease among patients
with malignancy versus those without malignancy: risk analysis using Medicare
claims data. Medicine. 1999;78:285-291.
6. Clinical Practice
Guidelines in Oncology: Venous Thromboembolic Disease, version 2.2006.
National Comprehensive Cancer Network Web site. Available at:
www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed April 8, 2007.
7. Merck & Co., Inc.
Elspar package insert. Whitehouse Station, NJ, December 2005.
8. Geerts WH, Pineo GF,
et al. Prevention of venous thromboembolism: The Seventh ACCP Conference on
Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338-400.
9. Leonardi MJ, McGory
ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer
patients: implications for improving quality. Ann Surg Oncol.
2007;14:929-936.
10. Buller HR,
Giancarlo A, et al. Antithrombotic therapy for venous thromboembolic disease:
The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.
Chest. 2004;126:401-428.
11. Verso M, Agnelli G,
et al. Enoxaparin for the prevention of venous thromboembolism associated with
central vein catheter: a double-blind, placebo-controlled, randomized study in
cancer patients. J Clin Oncol. 2005;23:4057-4062.
12. Couban S, Goodyear
M, et al. Randomized placebo-controlled study of low-dose warfarin for the
prevention of central venous catheter-associated thrombosis in patients with
cancer. J Clin Oncol. 2005;23:4063-4069.
13. Meyer G, Marjanovic
Z, et al. Comparison of low-molecular-weight heparin and warfarin for the
secondary prevention of venous thromboembolism in patients with cancer.
Arch Intern Med. 2002;162:1729-1735.
14. Deitcher SR,
Kessler CM, et al. Secondary prevention of venous thromboembolic events in
patients with active cancer: enoxparin alone versus initial enoxparin followed
by warfarin for a 180-day period. Clin Appl Thromb Hemost.
2006;12:389-396.
15. Hull RD, Pineo GF,
et al. Long-term low-molecular-weight heparin versus usual care in
proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:
1062-1072.
16. Lee AY, Levine MN,
et al. Low-molecular-weight heparin versus a coumarin for the prevention of
recurrent venous thromboembolism in patients with cancer. N Eng J Med.
2003;349;146-153.
17. Niers TMH, Klerk
CPW, et al. Mechanisms of heparin induced anti-cancer activity in experimental
cancer models. Crit Rev Oncol Hematol. 2007;61:195-207.
18. Lee AY, Rickles FR,
et al. Randomized comparison of low molecular weight heparin and courmarin
derivatives on the survival of patients with cancer and venous
thromboembolism. J Clin Oncol. 2005;23:2123-2129.
19. Kakkar AK, Levine
MN, et al. Low molecular weight heparin, therapy with dalteparin, and survival
in advanced cancer: the Fragmin Advanced Malignancy Outcome Study. J Clin
Oncol. 2004;22:1944-1948.
20. Klerk CPW,
Smorenburg SM, et al. The effect of low molecular weight heparin on survival
in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135.
To comment on this article,
contact editor@uspharmacist.com.






