US Pharm. 2010;35(10):10.
The FDA’s Center for Drug Evaluation and Research (CDER) ensures that marketed drugs are safe and effective. To market a new drug or biologic product, a company submits a New Drug Application (NDA) or Biologic License Application (BLA) to the FDA. A team of CDER/Center for Biologics Evaluation & Research physicians, statisticians, medicinal chemists, pharmacologists, and other scientists reviews the NDA or BLA. Since 2004, CDER has been reporting the number of BLAs and original NDAs it approves each month. In its report are listed the new molecular entities (NMEs), new formulations, and new combinations approved by the FDA.
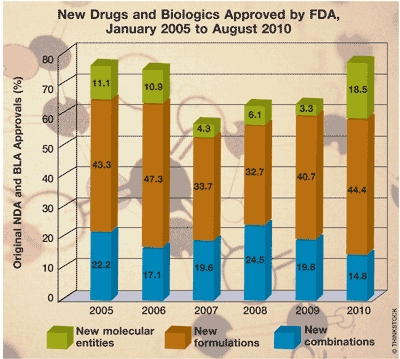
NMEs: The chart shows a declining trend in the number of NMEs the FDA has approved. Manufacturers’ inability to keep up the momentum of marketing innovative breakthrough drug products—possibly because of a dearth of research ideas—gave rise to the marketing of “me-too” products with marginal clinical benefit. Research dollars needed to undertake preclinical and clinical studies continue to rise, causing some concern about return on investment. Some manufacturers have been focusing on the development of drugs and biologics for the treatment or mitigation of cancer and other diseases. This process takes a long time, however. In the first quarter of 2010, the FDA approved six NMEs and one vaccine.
New Combinations: There has been a gradual increase in the marketing of drug products with multiple active pharmaceutical ingredients (APIs). Historically, U.S. consumers have predominantly been prescribed one pill for the treatment of each disease. With the aging of the population and the onset of multiple diseases, consumers are becoming noncompliant. Missed doses, doubled doses, and failure to remember to take the right drug at the right time have provoked concern among health care providers, as has consumers’ confusion about “look-alike” and “sound-alike” drugs. The recent increase in the number of new combination drugs and biologics has led health care providers to hope that patients will better adhere to their therapeutic regimens. The number of new combinations approved in the first 8 months of 2010 is at an all-time high.
New Formulations: A significant number of the new drugs and biologics approved by the FDA are new formulations. Such formulations include targeted drug delivery of APIs. As scientific knowledge expands, formulators continue to pursue improvement in the effectiveness of drugs and biologics. Consecutive years of sustained new drug output are needed. During 2010, licensing arrangements for drug candidates have been favored over industry consolidation.
To comment on this article, contact rdavidson@uspharmacist.com.






