US Pharm. 2015;40(4):HS2-HS6.
ABSTRACT: Hepatitis C virus (HCV) is the leading bloodborne chronic infection in the United States, with 3.2 million individuals believed to be infected. Injectable alpha interferons have historically been a mainstay of HCV therapy; however, they are only moderately effective and are challenging in terms of tolerability. Recent advances have led to the availability of interferon-free, all-oral, direct-acting antiviral medications that have made HCV therapy more efficacious and have simplified treatment regimens. These treatment regimens remain complicated, however, and guideline-based recommendations are continuing to evolve rapidly. The pharmacist should consult consensus HCV guidelines frequently in order to keep abreast of the most current recommendations for managing this patient population.
Hepatitis C virus (HCV) remains the leading bloodborne chronic infection in the United States.1 While there has been a general decline in the incidence of new cases since the early 1990s, the past few years have seen an uptick in the number of new cases reported. It is widely acknowledged, however, that HCV is significantly underreported for a variety of reasons, and the number of persons thought to be living with chronic HCV in the U.S. is estimated at 3.2 million. Further, more than 50% of infected individuals may not be aware of their HCV status.2 Approximately 20% of patients have symptomatic acute hepatitis, and 85% of these patients will develop chronic HCV (detectable virus for 6 months).3 The ongoing liver inflammation from chronic HCV is associated with an increased risk of liver fibrosis or cirrhosis, as well as hepatocellular carcinoma (HCC).4
VIROLOGY
HCV is a single-stranded, enveloped, RNA virus of the family Flaviviridae.5 Viral replication takes place primarily in the cytoplasm of hepatocytes and may also occur in peripheral blood cells. The viral genome undergoes cytoplasmic translation into a single polypeptide, which is then cleaved into 10 viral proteins. There are three structural and 10 nonstructural proteins, with the nonstructural proteins being the new antiviral targets.6 HCV RNA-dependent RNA polymerase is not highly accurate, and the virus undergoes frequent mutation during the replication process.7
There are seven known genotypes of HCV (genotypes 1-7).8 Genotype has an impact on the selection of initial therapy, and the treatment response varies by genotype. Genotype 1 is the most common cause of HCV in the U.S. Genotype 1 may be further subtyped into genotypes 1a and 1b, which have shown variable response by treatment.9,10
HCV is transmitted by exposure to infected blood products. The risk factors for virus acquisition are injection drug use, receipt of contaminated blood products, needlesticks (in healthcare workers), and vertical transmission. Approximately 85% of untreated infections progress to chronic HCV, and long-term sequelae of HCV infection include cirrhosis and HCC. Currently, cirrhosis related to HCV is the leading reason for liver transplantation in the U.S.11 Unlike the case with some other hepatitis viruses (e.g., hepatitis A and B), there is currently no vaccine available for HCV.
TESTING RECOMMENDATIONS AND TEST INTERPRETATION
The CDC recommends that HCV testing be performed in all persons born between 1945 and 1965.2 Additionally, anyone who is at increased risk for acquiring HCV because of high-risk behavior and/or exposure should be tested at least one time. Serology screening may be used to determine the presence of HCV antibodies, or an assay that detects HCV RNA may be employed. The HCV antibody test is the recommended initial screening mechanism for the vast majority of patients. HCV antibody testing may yield false-negative results, and a repeat test or HCV RNA testing should be considered in patients with recent HCV exposure (i.e., <6 months) and/or immunocompromising conditions.12 For patients in whom antiviral therapy will be initiated, quantitative HCV RNA testing is recommended to establish a baseline level of viremia prior to therapy.12 Patients with HCV should be evaluated for hepatitis B and HIV. Patients should also be assessed for the presence of cirrhosis, as this will determine the need for additional screening, such as for HCC.9 The interpretation of HCV test results is outlined in TABLE 1.
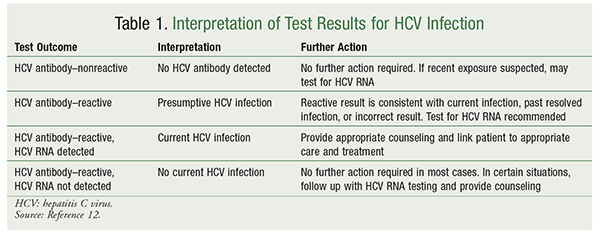
The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA), in collaboration with the International Antiviral Society–USA (IAS–USA), recommend treatment for all HCV-infected individuals, excepting those not expected to have long-term survival owing to non-HCV comorbidities.9 This guidance, which is based on evidence for treatment with recently approved oral anti-HCV medications, is a change from previous recommendations, which were limited by the number of viable treatment options and their toxicities and poor tolerability. Immediate initiation of antiviral therapy is suggested for patients with cirrhosis, advanced fibrosis, extrahepatic HCV manifestations, or previous liver transplantation.9 Laboratory testing, which is recommended prior to initiation of treatment, includes HCV RNA levels (including HCV genotype), CBC, liver function tests (LFTs), and markers of kidney function. See TABLE 1.
TREATMENT APPROACH
Early medication options for HCV included injectable alpha interferons, later combined with oral ribavirin to help improve outcomes.9 A proliferation of new oral antivirals that act directly against HCV have come to market in the past few years, and many other therapies are currently undergoing evaluation in the drug development pipeline. In addition to their improved tolerability, efficacy, and safety,9 these newer agents have simplified treatment regimens and shortened the duration of therapy, thereby creating a paradigm shift in how HCV is managed.
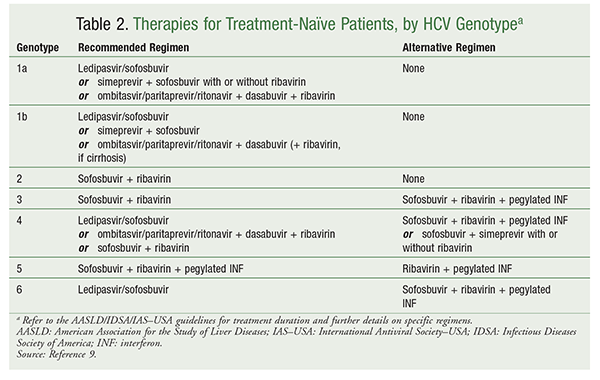
Treatment guidelines by HCV genotype for treatment-naïve patients are given in TABLE 2. For recommendations for treatment-experienced patients, refer to the AASLD/IDSA/IAS–USA guidelines.9 Treatment goals include eradication of HCV RNA and demonstration of a sustained virologic response (SVR). SVR, which is defined as undetectable levels of HCV RNA for at least 12 weeks following treatment completion, is a demonstrated marker of cure.13 Recent studies show that 99% of patients achieving SVR will have a prolonged response for at least 5 years.13,14 Other long-term goals include reduction of all-cause mortality, liver-related adverse events (AEs), and progression to end-stage liver disease and/or HCC.
TYPES OF TREATMENTS
Currently, two indirect-acting antivirals (IAAs) and seven direct-acting antivirals (DAAs) are approved for the treatment of chronic HCV infection in the U.S. The recommended treatment regimen depends upon the genotype (TABLE 2). The length of treatment is based on the treatment regimen and patient-specific factors such as previous experience with HCV treatment and presence of cirrhosis.9 The pharmacist can play an important role by assisting in the selection of the appropriate regimen based on patient-specific factors and taking a thorough medication history to assess for prior HCV therapies. Owing to the potential for drug-drug interactions (DDIs), maintenance medications may need to be modified, at least for the duration of HCV treatment. Because of the expense of treatment, clinicians must often obtain prior authorization from the patient’s insurance company before therapy initiation. Pharmacists should be involved in monitoring laboratory parameters and patient adherence to therapy. This article is meant as an overview, and the reader is therefore referred to the package insert for each agent and the AASLD/IDSA/IAS-USA guidelines for more specific information.
IAAs: Pegylated Interferon (IFN) Alfa-2a/b and Ribavirin
Alpha interferons induce immune response against HCV, which inhibits viral replication.15,16 Ribavirin is an oral guanosine analogue with activity against several RNA and DNA viruses.17 The exact mechanism of action of ribavirin against HCV is unclear. IFN and ribavirin have historically been the cornerstone of HCV therapy; however, these agents have had limited therapeutic success and have been largely replaced by DAAs.18,19 AEs include flulike symptoms, neutropenia, anemia, thrombocytopenia, depression, and thyroid dysfunction.15-17 Because of their severe side effects, low success rates, and long duration of therapy, IFN and ribavirin are currently recommended only as an alternative treatment for specific genotypes and in combination with at least one DAA.9,19
DAAs
Following the approval of DAAs, treatment success rates greatly increased, and treatment duration decreased.20 These agents directly target the HCV virus, particularly the nonstructural (NS) proteins. DAAs were originally added to treatment regimens with IFN and ribavirin, and many of the newer agents have facilitated IFN-free regimens.9
NS3/4A Protease Inhibitors (PIs): NS3/4A PIs target the serine protease NS3/NS4, which is responsible for processing HCV polyprotein and producing new viruses. Boceprevir (Victrelis) and telaprevir (Incivek), the first DAAs to be approved, are considered first-generation agents. Both medications are associated with long treatment duration, severe AEs, significant DDIs, and low treatment success rates compared with newer agents.9,21,22 Therefore, these agents are no longer recommended, and both of them have been removed from the market.9
Simeprevir (Olysio) is currently recommended as first-line treatment in conjunction with other agents.23 The most common AEs in clinical trials were rash (including photosensitivity), pruritus, nausea, myalgia, and dyspnea.23 It is recommended to test for the NS3 Q80K polymorphism prior to treatment. Simeprevir is associated with multiple DDIs, as it is a substrate and inhibitor of CYP1A2, CYP3A4 (intestinal), organic anion-transporter (OATP)1B1/3, and P-glycoprotein (PGP).23 This may have implications for patients coinfected with HIV.23,24
Paritaprevir (boosted with ritonavir) is also recommended as first-line treatment in conjunction with other agents in the Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets copackaged) and combined with ribavirin, if appropriate based on genotype. AEs for this combination therapy include nausea, pruritus, insomnia, and asthenia (all seen more frequently in patients requiring ribavirin).25 Paritaprevir is a substrate of PGP, breast cancer resistance protein (BCRP), CYP3A4, OATP1B1/3, and an inhibitor of uridine diphosphate-glucuronosyltransferase (UGT)1A1, OATP1B1/3, and BRCP. The potential for DDIs should be carefully determined in patients coinfected with HIV, as the use of darunavir, lopinavir/ritonavir, and rilpivirine is not recommended with paritaprevir.25
Nonstructural Protein 5A (NS5A) Inhibitors: NS5A inhibitors suppress the NS5A protein, which is essential for viral assembly and replication. Ledipasvir, which is licensed in combination with sofosbuvir (Harvoni), is recommended as first-line therapy. The treatment duration is dependent upon the baseline HCV viral load, the presence of cirrhosis, and previous HCV treatment experience.26 AEs include fatigue, headache, nausea, diarrhea, and insomnia. Ledipasvir inhibits PGP and BCRP and is a substrate of PGP and BCRP.26 It is important to be cognizant of potential problems with acid-reducing agents (e.g., antacids, histamine2-receptor antagonists, and proton pump inhibitors), as these products are available OTC and patients may potentially self-treat with them during HCV therapy.26 According to the package insert, patients coinfected with HIV may remain on certain antiretroviral agents, including atazanavir/ritonavir, darunavir/ritonavir, efavirenz, and rilpivirine, during HCV therapy.26
Ombitasvir is recommended as first-line treatment in conjunction with other agents in the Viekira Pak and ribavirin, if appropriate based on genotype. AEs for this combination therapy include nausea, pruritus, insomnia, and asthenia (all seen more frequently in patients requiring ribavirin).25 Ombitasvir is an inhibitor of UGT1A1 and a substrate of PGP and BCRP. It is mainly metabolized by amide hydrolysis; CYP enzymes play a minor role.25 The potential for DDIs should be carefully assessed in patients coinfected with HIV, as the use of darunavir, lopinavir/ritonavir, and rilpivirine is not recommended.25
Nonstructural Protein 5B (NS5B) Inhibitors: NS5B inhibitors suppress the NS5B RNA–dependent RNA polymerase, which is responsible for HCV replication. Sofosbuvir (Sovaldi) is recommended as first-line therapy for multiple genotypes in combination with other agents or ribavirin. AEs reported in IFN-free regimens (that included ribavirin) are fatigue, headache, nausea, insomnia, pruritus, anemia, asthenia, and rash.27 Sofosbuvir is a substrate of PGP and BCRP; however, its main inactive metabolite, GS-331007, is not. Neither sofosbuvir nor its metabolite is an inducer or substrate of CYP enzymes or UGT.24,27
Dasabuvir is recommended as first-line treatment in conjunction with other agents in the Viekira Pak and ribavirin, if appropriate based on genotype. AEs for this combination therapy include nausea, pruritus, insomnia, and asthenia (all seen more frequently in patients requiring ribavirin).25 Dasabuvir is an inhibitor of UGT1A1 and BCRP and a substrate of CYP2C8, PGP, and BCRP.25 The potential for DDIs should be carefully noted in patients coinfected with HIV, as the use of darunavir, lopinavir/ritonavir, and rilpivirine is not recommended.25
MONITORING, COUNSELING, AND NONPHARMACOLOGIC CONSIDERATIONS
Stringent monitoring of treatment efficacy, AEs, medication adherence, and DDIs is recommended for all patients undergoing antiviral therapy for HCV. Four weeks after therapy initiation, CBC, LFTs, and serum creatinine should be assessed. Quantitative tests for HCV RNA are recommended at weeks 4 and 12 and may be considered at week 24 (after completion of therapy).9 Thyroid-stimulating hormone levels should be evaluated at the beginning and end of therapy if IFN-based treatment is administered. If ribavirin is being considered in women of childbearing age, a serum pregnancy test prior to treatment initiation is recommended.
Prior to therapy initiation, patients should be extensively educated regarding their HCV medications, monitoring plan, and reduction of transmission or reinfection. Patients should be cautioned to avoid the use of alcohol, which can lead to further liver decompensation, including progression of cirrhosis or development of end-stage liver disease.28 Once therapy has started, patients should be monitored by telephone or in person for medication adherence. Patients should be counseled to report any new prescription or OTC medications to assess for new DDIs during therapy or the potential for new medications to cause hepatotoxicity. Other nonpharmacologic considerations include vaccination for hepatitis A and hepatitis B in susceptible patients.9
CONCLUSION
Recent developments in the availability of highly efficacious, well-tolerated HCV drug regimens have significantly changed the medical management of HCV. Treatment has evolved from longer-term immune-based therapies—which are moderately effective and rife with toxicities—to newer and more efficacious therapies demonstrating higher success rates, fewer AEs, and shorter durations of therapy. As these options become increasingly more accessible, it is possible that HCV may one day become routinely curable. Treatment regimens remain complicated, however, and guideline-based recommendations continue to evolve rapidly. The reader is strongly urged to refer to consensus HCV guidelines frequently in order to remain apprised of the most current recommendations for managing this patient population.
REFERENCES
1. CDC. Disease burden from viral hepatitis A, B, and C in the United States. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf. Accessed January 15, 2015.
2. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
3. Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31(suppl 1):9-16.
4. Zoulim F, Chevallier M, Maynard M, Trepo C. Clinical consequences of hepatitis C virus infection. Rev Med Virol. 2003;13:57-68.
5. Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000;81:1631-1648.
6. Moradpour D, Penin F. Hepatitis C virus proteins: from structure to function. Curr Top Microbiol Immunol. 2013;369:113-142.
7. Ribeiro RM, Li H, Wang S, et al. Quantifying the diversification of hepatitis C virus (HCV) during primary infection: estimates of the in vivo mutation rate. PLoS Pathog. 2012;8:e1002881.
8. Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
9. AASLD/IDSA/IAS–USA. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org. Accessed January 6, 2015.
10. Cortez KJ, Kottilil S. Beyond interferon: rationale and prospects for newer treatment paradigms for chronic hepatitis C. Ther Adv Chronic Dis. 2015;6:4-14.
11. Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521.
12. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365.
13. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
14. Manns MP, Pockros PJ, Norkrans G, et al. Long-term clearance of hepatitis C virus following interferon a-2b or peginterferon a-2b, alone or in combination with ribavirin. J Viral Hepat. 2013;20:524-529.
15. Pegintron (peginterferon alfa-2b) product information. Whitehouse Station, NJ: Merck & Co, Inc; 2013.
16. Pegasys (peginterferon alfa-2a) product information. South San Francisco, CA: Genentech USA, Inc; September 2014.
17. Copegus (ribavirin) product information. South San Francisco, CA: Genentech USA, Inc; February 2013.
18. Mangia A, Santoro R, Minerva N, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609-2617.
19. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965.
20. Chung RT, Baumert TF. Curing chronic hepatitis C—the arc of a medical triumph. N Engl J Med. 2014;370:1576-1578.
21. Viktrelis (boceprevir) product information. Whitehouse Station, NJ: Merck & Co, Inc; 2013.
22. Incivek (telaprevir) product information. Cambridge, MA: Vertex Pharmaceuticals Inc; October 2013.
23. Olysio (simeprevir) product information. Titusville, NJ: Janssen Therapeutics; November 2014.
24. Karageorgopoulos DE, El-Sherif O, Bhagani S, Khoo SH. Drug interactions between antiretrovirals and new or emerging direct-acting antivirals in HIV/hepatitis C virus coinfection. Curr Opin Infect Dis. 2014;27:36-45.
25. Viekira Pak (ombitasvir/paritaprevir/ritonavir and dasabuvir) product information. North Chicago, IL: AbbVie Inc; February 2015.
26. Harvoni (ledipasvir/sofosbuvir) product information. Foster City, CA: Gilead Sciences, Inc; October 2014.
27. Sovaldi (sofosbuvir) product information. Foster City, CA: Gilead Sciences, Inc; November 2014.
28. Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171.
To comment on this article, contact rdavidson@uspharmacist.com.






