US Pharm. 2015;40(11)(Oncology suppl):3-6.
ABSTRACT: Melanoma is a leading cause of skin cancer mortality in the United States. Treatment for melanoma had been limited to very few agents, including chemotherapy, with older treatment options such as interferon and interleukin-2 causing toxic adverse effects. Since the approval of the monoclonal antibody ipilimumab in 2011, there have been several new drugs approved for metastatic melanoma. Recently, there have been approvals of kinase inhibitors and programmed death receptor-1 (PD-1) inhibitors for unresectable or metastatic melanoma, including vemurafenib, nivolumab, and pembrolizumab. These agents have been considered breakthrough therapies in the treatment of melanoma.
Melanoma is one of the leading cancer sites and is increasingly common in the United States.1,2 Melanoma is also the most common cause of skin cancer mortality.3 The incidence of melanoma continues to increase more rapidly than any other cancer in men, at an overall rate of 33%.4 In women, the incidence of melanoma is increasing more rapidly than that of any other malignancy except lung cancer, at an overall rate of 23%.4 Even with the reported increases, these figures may underestimate the true incidence because many in situ melanomas that are outpatient-treated are undocumented. At 82% to 85%, the vast majority of melanomas present as localized disease, 10% to 13% as regional, and 2% to 5% as distant metastatic disease.4 The earlier the malignancy is caught, the more favorable the outcome.
Treatment Overview
Primary early-stage melanoma is highly curable by surgical resection; however, treatment of metastatic or advanced disease requires chemotherapeutic agents.4 As with any therapy, the risks and benefits of interferon alfa-2b and interleukin-2 therapy are assessed prior to starting treatment. Alkylating chemotherapy agents such as dacarbazine and temozolomide are used in treating advanced melanoma. Temozolomide does not have a FDA approval for metastatic melanoma, but it is commonly used in practice. The oral route of administration of temozolomide, as well as its ability to penetrate the central nervous system (CNS), makes it an appealing single agent or combination therapy option in metastatic malignancies. Dacarbazine is the only FDA-approved treatment for metastatic melanoma that is used as a single agent, IV only.5-8
The monoclonal antibody ipilimumab was approved for the treatment of unresectable or meta-static melanoma in 2011. At the time, ipilimumab was the first new treatment for melanoma in over a decade and was quickly approved using fast-track designation due to its benefit of prolonging survival.9 Since then, there have been several new drugs approved for melanoma, and this article will focus on giving pharmacists a review of these newer emerging therapies, especially kinase inhibitors and programmed death receptor-1 (PD-1) inhibitors.
Kinase Inhibitors
The introduction of tyrosine kinase inhibitors for the treatment of unresectable or metastatic melanoma has led to greatly improved response and overall survival (OS) rates in patients with targetable kinase mutations.10 The kinase inhibitors vemurafenib (see below) and dabrafenib are approved for the treatment of BRAF-positive advanced melanoma. The BRAF gene in humans is involved in cellular signaling and is mutated in some cancers. Having a therapy directed at this gene mutation has been shown to improve progression-free survival (PFS) and OS in patients with BRAF V600 mutations. However, resistance develops in many patients using BRAF-targeted therapy due to mutations in the MAPk pathway. To overcome this resistance, MEK inhibitors were developed. Trametinib, an MEK-1 inhibitor, was approved in 2013 for BRAF-mutated metastatic melanoma. A study by Long et al demonstrated that the combination of the BRAF kinase inhibitor dabrafenib and the MEK-1 inhibitor trametinib improved the rate of PFS and OS in previously untreated patients who had metastatic melanoma with BRAF V600E or V600K mutations.11
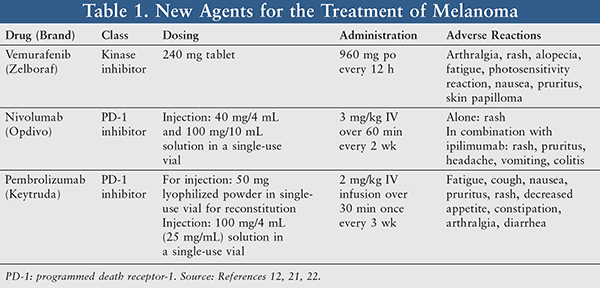
Vemurafenib: Vemurafenib (Zelboraf) is a kinase inhibitor indicated for the treatment of patients with unresectable stage IIIC or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test. Before starting vemurafenib, a dermatologic evaluation, baseline monitoring of ECG, electrolytes, liver enzymes, and bilirubin should be performed. Standard dosing is 960 mg orally every 12 hours, with treatment continuing until disease progression or unacceptable toxicity occurs. No renal or hepatic dose adjustments are required, no contraindications to therapy have been identified, and the drug is listed as Pregnancy Category D. Vemurafenib is supplied as 240 mg tablets that can be taken with or without a meal. The tablet should not be crushed or chewed, and missed doses can be taken up to 4 hours prior to the next dose (TABLE 1).12
Concomitant use with other agents known to prolong QT interval and drugs predominantly metabolized by CYP1A2 that have a narrow therapeutic window is not recommended. Use with strong CYP3A4 inhibitors or inducers should be avoided; however, if coadministration is necessary, monitor closely for toxicities and consider dose reduction of CYP1A2 substrates. Patients should be instructed to avoid sun exposure, wear protective clothing, use a sunscreen, and immediately report symptoms of severe dermatologic reactions or new cutaneous malignancies, any cardiac symptoms, vision changes, photophobia, or symptoms of uveitis. The price for 120 tablets, which is a 1-month supply of vemurafenib, is $6,510.48.12-14
Trials that established efficacy and approval of vemurafenib include an international, open-label, randomized, controlled study of 675 patients with treatment-naïve, BRAF V600E mutation–positive unresectable or metastatic melanoma.12,15 Patients were randomized to receive vemurafenib 960 mg by mouth twice daily or dacarbazine 1,000 mg/m2 IV on day 1 every 3 weeks. Treatment continued until disease progression, unacceptable toxicity, and/or consent withdrawal occurred. The major efficacy outcome measures of the trial were OS and investigator-assessed PFS. At 6 months, OS was 84% in the vemurafenib group and 64% in the dacarbazine group. In the interim analysis for OS and final analysis for PFS, vemurafenib was associated with a relative reduction of 63% in the risk of death and of 74% in the risk of either death or disease progression, as compared with dacarbazine (P <.001 for both comparisons).12,15
Overall, vemurafenib has shown consistent results for patients with BRAF V600E–mutated metastatic melanoma in several trials, and the adverse effects are no more severe than those of other standard chemotherapy agents. Caution should be used in patients with impaired pancreatic function, and additional trials should be conducted to see the exact mechanism and association this drug has for causing pancreatitis. Aside from this, vemurafenib is a good choice for BRAF-positive patients with advanced melanoma. This drug could also be a treatment option in the future for many other cancers and disease states based on the significant number of trials that are currently being conducted. There are currently 99 active, ongoing clinical trials using vemurafenib in many other types of cancer and disorders, such as blood coagulation disorders and endocrine system diseases.16 Of course, the results of these trials will need to be thoroughly assessed and more studies are required to establish vemurafenib’s efficacy and place in therapy for other diseases.17-19
PD-1 Inhibitors
A new class of drugs, called PD-1 inhibitors, are part of the evolving monoclonal antibody immuno-therapy treatment options for metastatic disease. PD-1 inhibitors were developed to overcome the inhibition of the adaptive immunity response by PD-1–positive cancer types. Melanoma tumor cells express the immunosuppressive PD-1 ligand. By blocking the binding of the inhibitory PD-1 ligand to the PD-1 receptor on tumor cells, the host’s immune system is actively stimulated, and T cells can work more effectively.20 The PD-1 inhibitors nivolumab and pembrolizumab were recently approved in July and September 2014, respectively (TABLE 1).21,22
Nivolumab: Nivolumab (Opdivo) is a human immunoglobulin G4 (IgG4) and PD-1 inhibitor approved for the treatment of melanoma and non–small cell lung cancer (NSCLC).2,21 The current FDA indications include unresectable or metastatic melanoma and metastatic squamous NSCLC following failure of first-line medications. Nivolumab works by blocking the interaction of PD-1 and its ligand, which are involved in the negative regulation of the T-cell cycle. Blockage of this interaction results in the activation/proliferation of T cells and the enhancement of the antitumor response.21,23
The recommended dose of nivolumab is 3 mg/kg administered IV over 60 minutes every 2 weeks, and therapy is continued indefinitely until disease progression or intolerable toxicities occur.21 If the patient is experiencing any renal toxicity, hepatic toxicity, colitis, or pneumonitis of severity grades 2 to 3, therapy should be held and a corticosteroid administered. For toxicities of grades 3 to 4, discontinue therapy.23 Due to the mechanism of action, the significant adverse drug reactions are immune-mediated and include pneumonitis, colitis, hepatitis, nephritis, hyper- or hypothyroidism, and other effects.21 The cost of a 40-mg/4-mL vial is $1,151.04, and the cost for a vial of 100 mg/10 mL is $2,877.60.23
The safety of nivolumab was studied in a phase III, randomized, open-label trial that included 370 patients with unresectable or metastatic melanoma.24 The patients were given either nivolumab 3 mg/kg every 2 weeks or investigator’s choice chemotherapy (ICC; dacarbazine 1,000 mg/m2 every 3 weeks or combination carboplatin AUC 6 plus paclitaxel 175 mg/m2 every 3 weeks). This trial demonstrated improved duration of response in the nivolumab arm with tolerable adverse drug reactions compared to the ICC arm. The interim analysis of the first 120 patients who received nivolumab evaluated the efficacy and found an objective response rate of 32% (95% CI, 23-41).24
In conclusion, nivolumab is a novel PD-1 inhibitor currently used in the treatment of metastatic or unresectable melanoma and metastatic NSCLC. This agent works by blocking the negative regulation of T cells and enhancing the antitumor response.21,23 Previous trials have compared nivolumab versus chemotherapy regimens for melanoma and NSCLC. The phase III randomized trial for patients with metastatic or unresectable melanoma showed that nivolumab had a better overall response rate (ORR) with tolerable toxicities compared to that of ICC.24
Pembrolizumab: Pembrolizumab (Keytruda) was granted breakthrough therapy designation in January 2013 based on preliminary evidence of clinical activity. It was approved by the FDA on September 4, 2014, on condition that its manufacturer, Merck Sharp & Dohme Corp, conducted a multicenter, randomized trial to establish the superiority over standard therapy verifying and describing the clinical benefits. Pembrolizumab is a highly selective humanized monoclonal antibody that binds to the PD-1 receptor on T cells, blocking PD-1 and PD-2 ligands from binding, and inhibiting negative immune regulation, including antitumor immune response.25
Pembrolizumab is used in treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and a BRAF inhibitor (if BRAF V600 mutation–positive).26 It is dosed at 2 mg/kg IV infusion over 30 minutes once every 3 weeks until progression or unacceptable toxicity occurs in adult and geriatric patients.26,27 It comes as a lyophilized powder for injection in doses of 100 mg/4 mL for $5,179.20 and 50 mg for $2,589.60; once diluted, it can last 6 hours at room temperature and 24 hours from time of dilution if refrigerated (36ºF-46ºF).26
Treatment should be withheld at aspartate/alanine aminotransferase (AST/ALT) >3 to 5 times upper limit of normal (ULN) or total bilirubin >1.5-3 times ULN; grade 2 pneumonitis and nephritis; grade 2 or 3 colitis; grade 3 hyperthyroidism and treatment-related adverse reaction; and symptomatic hypophysitis. Permanently discontinue at AST/ALT >5 times ULN or total bilirubin >3 times ULN; any life-threatening adverse reaction; grade 3 or 4 pneumonitis, nephritis, and infusion-related reactions; corticosteroid of >10 mg of prednisone or equivalent per day within 12 weeks; persistent grade 2 or 3 adverse reactions not recovering to grade 0 or 1 within 12 weeks after last dose; and any severe grade-3 reaction that recurs.22
Pembrolizumab is Pregnancy Category D, and use of contraception until 4 months after taking the last dose is recommended. There is no information on overdose, and pembrolizumab has not been studied for drug interactions, contraindications, and estimated glomerular filtration rate (eGFR) <15 mL/min, or in nursing mothers and pediatric patients; therefore; it should not be used in these populations. Resulting immune-mediated conditions include pneumonitis, colitis, hepatitis, hypophysitis, nephritis, hyperthyroidism, and adverse reactions. However, fatigue, chills, nausea/vomiting, diarrhea, constipation, cough, dyspnea, rash, decreased appetite, arthralgia, anemia, pain, headache, dizziness, and insomnia occur in >10% of patients. Therefore, immune-mediated reactions should be monitored and all precautions taken.22,27
The KEYNOTE-006 trial compared pembrolizumab with ipilimumab and further confirmed the effectiveness of the 3-week dosing interval and also elucidated the prolonged PFS and OS with less high-grade toxicity achieved with pembrolizumab in advanced melanoma. However, postmarketing surveillance will ascertain the safety and efficacy of pembrolizumab in therapy, which is currently under way.28-30
Conclusion
In conclusion, metastatic melanoma is an advanced skin cancer that until recently had limited treatment options. Pharmacists play a significant role in helping providers and patients choose the best therapy based on toxicities and cost, along with providing counseling. Newer therapies such as kinase and PD-1 inhibitors have shown impressive clinical responses in advanced melanoma.
REFERENCES
1. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2012 Incidence and Mortality Web-based Report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. www.cdc.gov/uscs. Accessed July 16, 2015.
2. FDA news release. FDA approves Opdivo for advanced melanoma. December 22, 2014. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427716.htm. Accessed July 16, 2015.
3. Hauschild A. Adjuvant interferon alfa for melanoma: new evidence-based treatment recommendations? Current Oncol. 2009;16(3):3-6.
4. Melanoma National Comprehensive Cancer Network guidelines. Version 3.2015. www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed July 28, 2015.
5. Temodar (temozolomide) package insert. Tulsa, OK: Physicians Total Care, Inc; 2011.
6. Dacarbazine package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2012.
7. Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19(1):21-34.
8. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9): 2745-2751.
9. Fellner C. Ipilimumab (Yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. Pharm Ther. 2012;37(9):503-530.
10. Livingstone E, Zimmer L, Vaubel J, Schadendorf D. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin Clin Oncol. 2014;3(3):29 www.thecco.net/article/view/3807/5239. Accessed October 14, 2015.
11. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877-1888.
12. Zelboraf (vemurafenib) package insert. South San Francisco, CA: Genentech, Inc; 2014.
13. Vemurafenib. DRUGDEX. Micromedex [online database]. Greenwood Village, CO: Truven Health Analytics, Inc; 2013. www.micromedexsolutions.com. Accessed July 17, 2015.
14. Vemurafenib. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2015. http://online.lexi.com. Accessed July 17, 2015.
15. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
16. Vemurafenib. U.S. National Library of Medicine, U.S. National Institutes of Health, Department of Health and Human Services; 2015. www.clinicaltrials.gov. Accessed July 20, 2015.
17. Haroche J, Cohen-Aubart F, Emile JF, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411-418.
18. Pettirossi V, Santi A, Imperi E, et al. BRAF inhibitors reverse the unique molecular signature and phenotype of hairy cell leukemia and exert potent antileukemic activity. Blood. 2015;125(8): 1207-1216.
19. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867-1876.
20. Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014;21(3):231-237.
21. Opdivo (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb Company; March 2015.
22. Keytruda (pembrolizumab) package insert. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; September 2014.
23. Nivolumab. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; updated May 1, 2015. http://online.lexi.com. Accessed July 13, 2015.
24. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
25. FDA. Pembrolizumab. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm412861.htm. Accessed July 16, 2015.
26. Pembrolizumab. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2015. http://online.lexi.com. Accessed July 16, 2015.
27. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
28. Sullivan RJ, Flaherty KT. Pembrolizumab for treatment of patients with advanced or unresectable melanoma. Clin Cancer Res. 2015;21(13):2892-2897.
29. Stenger M. First approval of PD-1 inhibitor: pembrolizumab in unresectable or metastatic melanoma. ASCO Post. October 15, 2014;5(16). www.ascopost.com/issues/october-15,-2014/first-approval-of-pd-1-inhibitor-pembrolizumab-in-unresectable-or-metastatic-melanoma.aspx. Accessed July 16, 2015.
30. National Comprehensive Cancer Network. Melanoma (Version 3.2015). www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed July 19, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.





