US Pharm. 2016;41(12):20-23.
ABSTRACT: Over 29.1 million people in United States have diabetes and are faced with the complications associated with the disease. Diabetes is the most common systemic disease that causes gastroparesis. Diabetic gastroparesis is commonly suspected in poorly controlled diabetic patients who present with gastrointestinal complaints, especially following a meal. Once a diagnosis is made, management of the condition is centered on optimizing blood glucose control, providing nutritional support including hydration, and in many cases using prokinetic and antiemetic medications. As healthcare providers, community pharmacists are in key positions to assist patients in how to manage this irreversible complication of diabetes.
Approximately 9.3% of the U.S. population has diabetes.1 This equates to about 29.1 million people who could be visiting community pharmacies for diabetes medications and supplies.2 Diabetic patients may also seek pharmacists for education and advice on how best to maintain blood glucose control in hopes of avoiding complications such as cardio-vascular disease, stroke, nephropathy, and retinopathy. Among the many complications, diabetes is the most common systemic disease that causes gastroparesis.3
Diabetic gastroparesis is an autonomic neuropathic complication of diabetes not due to a mechanical gastrointestinal (GI) obstruction.4 Uncontrolled elevations of blood glucose over a long period of time can damage nerves in the enteric nervous system, which governs the GI system.5,6 Subsequently, the stomach and small intestine as well as the smooth muscle cells of the gut are unable to function properly. When enough cells are affected, impaired peristalsis or even gastric stasis can occur.7,8 Ultimately, this can lead to malnutrition, poor quality of life, and hospitalizations. In fact, according to Medicare-based data, the number of gastroparesis hospitalizations in the United States is on the rise.9
Epidemiologic studies vary widely, suggesting that diabetic gastroparesis may occur in anywhere from 1% up to 65% of patients with diabetes.10-14 Nevertheless, it is known to be most prevalent in type 1 diabetes mellitus patients age >40 years with a 10-year history of diabetes.14 Furthermore, gastroparesis has been shown to be more prevalent among women.15 This article will review typical clinical characteristics along with potential management therapies of diabetic gastroparesis.
Clinical Manifestations and Diagnosis
Diabetic gastroparesis is commonly suspected in poorly controlled diabetic patients who present with complaints of nausea, vomiting, bloating, abdominal pain, early satiety, or postprandial fullness.16 Vomiting more often supports evaluation of patients for diabetic gastroparesis compared to other symptoms.3 Frequency, severity, and intensity of symptoms may vary over time in addition to varying among patients, making diagnosis challenging.17 Extreme cases of gastroparesis may lead to severe nausea, vomiting, starvation, weight loss, and dehydration requiring urgent care.3,17 Another potential complication of gastroparesis is the formation of a bezoar, a solid collection of material such as food, mucus, vegetable fiber, or hair that cannot be digested.17
Should a patient exhibit symptoms suggestive of diabetic gastroparesis, a thorough medical history and physical examination should be obtained. Further diagnostic testing should then proceed in an effort to rule out mechanical obstruction and confirm the presence of diabetic gastroparesis.3,17 There are a number of diagnostic tests that can be used to examine gastric motility. Scintigraphy, the gold standard, is performed 4 hours after ingestion of a solid, radiolabeled meal by measuring the extent of gastric retention.18,19 Gastric retention of >10% at 4 hours and/or >60% at 2 hours confirms gastroparesis.19 Radiographic images from CT scans or MRIs may be initially obtained at the time of presentation to help exclude mechanical obstruction. A recently FDA-approved diagnostic method is the 13C-Spirulina Gastric Emptying Breath Test (GEBT).20 Unlike scintigraphy, this test uses a nonradioactive isotope bound to solid food. After ingestion of the stable isotope-labeled test meal, the expiratory 13CO2 (carbon-13) concentration is measured by mass spectrometry to determine and measure gastric emptying. Alternatively, antroduodenal manometry may also be used to measure GI motility before and after eating. Other diagnostic methods not discussed include electrogastrography, ultrasonography, swallowed capsule telemetry, and an upper GI barium series.18,21-24
Management
Once a diagnosis is made, management of the condition is centered on optimizing blood glucose control and nutritional support, including hydration and prokinetic and antiemetic medications. In cases of refractory diabetic gastroparesis, surgical interventions may be taken such as gastric electrical stimulation, placement of a gastrostomy tube, or, in severe cases, gastrectomy. Refractory cases may also require the need for parenteral nutrition. Overall, selection of therapy is largely based on the severity of the condition, which is classified according to a patient’s ability to maintain adequate nutrition and responsiveness to therapy.25 Mild diabetic gastroparesis is usually characterized by intermittent symptoms, which easily respond to improvements in glycemic control or minor dietary adjustments.5 When symptoms are persistent, patients may be classified as having moderate or severe diabetic gastroparesis. In addition to maintaining glycemic control and dietary modifications, these patients often require medication(s) to increase gastric motility and may be occasionally hospitalized.5 Those who are unresponsive to medications are considered to have refractory gastroparesis.
It is important to understand that the aforementioned therapies do not cure diabetic gastroparesis, as it is a chronic and relapsing condition. Treatment instead helps patients maintain a more positive quality of life and prevent hospitalizations.17
Regardless of the severity of the gastroparesis, adequate blood glucose control is critical, as hyperglycemia directly interferes with normal stomach emptying as well as prokinetic drugs used to treat gastroparesis.26 However, glycemic control can be more difficult in diabetic patients with gastroparesis because food absorption is delayed and unpredictable, making blood glucose levels erratic.17 Patients should discuss with their providers strategies to improve glucose control such as taking insulin more often, changing the type of insulin they use, taking insulin after meals instead of before, or checking blood glucose levels more frequently after eating to determine when insulin admin-istration is necessary.27

Diet modifications are considered one of the initial steps in managing diabetic gastroparesis. Patients with mild disease should first be directed to avoid particular foods that may contribute to delayed emptying and participate in postprandial activities that promote gastric emptying (TABLE 1).3,18,21,28-30 Additionally, medications that inhibit GI motility should be discontinued (TABLE 2).18,21,24 Irrespective of the degree of symptoms, patients should maintain adequate hydration to minimize electrolyte disturbances and metabolic deficiencies due to vomiting and a decrease in oral intake. Vitamin supplementation should be recommended to those who are able to tolerate oral intake. If the patient is unable to tolerate oral food or if he or she becomes refractory to treatment medications, enteral or parenteral feeding may be considered. Enteral nutrition is indicated when patients have an unintentional loss of ≥10% of usual body weight in a period of 3 to 6 months.25 A regulated enteral nutrition may improve glycemic control, but may have complications including infection, tube migration, and dislodgement.3

Pharmacologic Treatment
As previously mentioned, when glycemic control and dietary changes do not attenuate symptoms, prokinetic agents may be prescribed (TABLE 3).21,24,31 Metoclopramide, the only FDA-approved medication for gastroparesis, is considered a first-line agent in the treatment of diabetic gastroparesis.3 Metoclopramide is a dopamine2 (D2)-receptor antagonist, 5-HT4 (serotonin)-receptor agonist, and 5-HT3-receptor antagonist that stimulates smooth muscle contraction.17 It also has an antiemetic effect.16 Meto-clopramide should be administered at the lowest effective dose for a maximum 12-week period unless the benefit outweighs the risk. Drug holidays may be employed whenever clinically possible due to the boxed warning of extrapyramidal side effects, specifically tardive dyskinesia.3,17 Although the risk of tardive dyskinesia is estimated to be <1%, patients should be counseled to discontinue the use of metoclopramide if involuntary movements develop.3,32 Compared to other prokinetic agents, metoclopramide is available in multiple dosage forms including oral tablet, oral disintegrating tablet, liquid, and parenteral (IM or IV). The liquid formulation is preferred, as it has improved absorption and better facilitates dose titrations and reductions.3
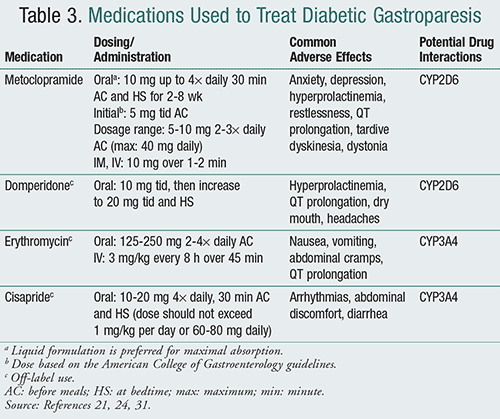
Domperidone, a peripheral D2-receptor antagonist, is not commercially available in the U.S. but can be obtained by a physician for patients with refractory gastroparesis though the Investigational New Drug (IND) application program at the FDA.33 Domperidone has demonstrated to be just as effective as metoclopramide in reducing symptoms without the central nervous system side effects.34 A baseline electrocardiogram should be obtained prior to domperidone initiation in case of QT prolongation.3 As with metoclopramide, tolerance can develop to the improvement by about 6 weeks.16,35
Patients who fail therapy with metoclopramide and domperidone may attempt to use erythromycin. Erythromycin, a macrolide antibiotic, is a motilin agonist that can increase gastric emptying and symptoms such as bloating for several weeks.24 Respon-siveness, however, is limited by tachyphylaxis, which can develop 4 weeks after starting therapy.3 Compared to oral erythromycin, IV erythromycin has shown a greater effect on gastric emptying.36 While other macrolides have been used, controlled clinical trials are lacking to support their use.16,37
Another drug no longer commercially available due to its proarrhythmic adverse effects is cisapride. However, it too may be acquired through the IND program at the FDA for patients with refractory gastroparesis. Cisapride is a 5-HT4-receptor agonist that speeds up gastric emptying and reduces symptoms associated with gastroparesis.16,31
Antiemetic agents may be used to assist with symptoms associated with diabetic gastroparesis but do not improve gastric emptying. Antihistamines, promethazine, and prochlorperazine are commonly prescribed first-line options that have been well studied. Second-line agents such as ondansetron and granisetron, 5-HT3 antagonists, have also shown efficacy but have not been compared to older antiemetics for gastroparesis.3 Low doses of the tricyclic antidepressants (TCAs) nortriptyline, amitriptyline, and doxepin have also been used but are considered off-label for those with refractory diabetic gastroparesis.3
Role of the Pharmacist
Diabetic gastroparesis is an irreversible complication of diabetes. Diabetic patients may present to the pharmacy with GI symptoms suggesting diabetic gastroparesis. Knowing possible self-care treatments to recommend to patients is important for community pharmacists. As the most accessible healthcare providers, community pharmacists are in key positions to educate patients on how to appropriately monitor their blood glucose and properly take their diabetic medications. It is important to understand that therapeutic recommendations will not cure their diabetic gastroparesis, but may help to slow further damage and avoid potential complications.
Community pharmacists have the opportunity to advise diabetic patients about lifestyle modifications, such as diet. Even though patients with diabetic gastroparesis may feel full and may not want to eat, obtaining adequate nutrition is still important. Pharmacists can recommend eating smaller, more frequent meals that are low in fat and fiber to prevent even slower gastric emptying. In more severe cases, suggest switching to a liquid diet, which could make digestion easier.
Some medications may exacerbate symptoms of diabetic gastroparesis. Pharmacists should remember to educate patients on the types of drugs that delay gastric emptying and to avoid them, if possible. If patients remain unresponsive to lifestyle modifications, pharmacists should refer patients to their physician, who may prescribe prokinetic and antiemetic medications to better control the symptoms of diabetic gastroparesis.
REFERENCES
1. American Diabetes Association. Statistics about diabetes. Updated April 1, 2016. www.diabetes.org/diabetes-basics/statistics. Accessed September 25, 2016.
2. Centers for Disease Control and Prevention. Diabetes home. Basics. Updated October 7, 2015. www.cdc.gov/diabetes/basics/index.html. Accessed September 25, 2016.
3. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
4. Malamood M, Parkman H, Schey R. Current advances in treatment of gastroparesis. Expert Opin Pharmacother. 2015;16:1997-2008.
5. Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820-829.
6. King MW. Digestion: anatomy, biochemistry, and physi-ology. In: King MW, ed. Integrative Medical Biochemistry Examination and Board Review. New York, NY: McGraw-Hill; 2014.
7. Bouras EP, Vazquez Roque MI, Aranda-Michel J. Gastroparesis from concepts to management. Nutr Clin Pract. 2013;28:437-447.
8. Al-Shboul OA. The importance of interstitial cells of Cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19:3-15.
9. Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103:313-322.
10. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuro-pathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293.
11. Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264-1269.
12. Kong MF, Horowitz M, Jones KL, et al. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22(3):503-507.
13. Horowitz M, Maddox AF, Wishart JM, et al. Relationships between esophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med. 1991;18(4):229-234.
14. Choung RS, Locke GR III, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82-88.
15. Dickman R, Wainstein J, Glezerman M, et al. Gender aspects suggestive of gastroparesis in patients with diabetes mellitus: a cross-sectional survey. BMC Gastroenterol. 2014;14:34-39.
16. Reddymasu SC, McCallum RW. Pharmacotherapy of gastroparesis. Expert Opin Pharmacother. 2009;10:469-484.
17. National Institute of Diabetes and Digestive and Kidney Diseases. Gastroparesis. Updated June 2012. www.niddk.nih.gov/health-information/health-topics/digestive-diseases/gastroparesis/Pages/facts.aspx. Accessed September 25, 2016.
18. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589-1591.
19. Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456-1462.
20. FDA approves breath test to aid in diagnosis of delayed gastric emptying. FDA news release. April 6, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm441370.htm. Accessed September 26, 2016.
21. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
22. Darwiche G, Bjorgell O, Thorsson O, Almer LO. Correlation between simultaneous scintigraphic and ultrasonographic measurement of gastric emptying in patients with type 1 diabetes mellitus. J Ultrasound Med. 2003;22:459-466.
23. Kuo B, McCallum R, Koch K, et al. SmartPill, a novel ambulatory diagnostic test for measuring gastric emptying in health and disease. Gastroenterology. 2006;130:A434.
24. Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther. 2008;27(9):724-740.
25. Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263-283.
26. Petrakis IE, Kogerakis N, Prokopaksi G, et al. Hyper-glycemia attenuates erythromycin-induced acceleration of liquid-phase gastric emptying of hypertonic liquids in healthy subjects. Dig Dis Sci. 2002;47:67-72.
27. American Diabetes Association. Gastroparesis. Updated June 27, 2014. www.diabetes.org/living-with-diabetes/complications/gastroparesis.html. Accessed September 25, 2016.
28. Wytiaz V, Homko C, Duffy F, et al. Foods provoking and alleviating symptoms in gastroparesis: patient experiences. Dig Dis Sci. 2015;60:1052-1058.
29. Homko CJ, Duffy F, Friedenberg FK, et al. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2015;27:501-508.
30. Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology. 2011;141:486-498.
31. Ali T, Hasan M, Hamadani M, Harty RF. Gastroparesis. South Med J. 2007;100:281-286.
32. Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31(1):11-19.
33. FDA. How to request domperidone for gastrointestinal disorders. Updated October 31, 2016. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/investigationalnewdrugindapplication/ucm368736.htm. Accessed November 9, 2016.
34. Patterson D, Abell T, Rothstein R, et al. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230-1240.
35. Gumaste V, Baum J. Treatment of gastroparesis: an update. Digestion. 2008;78:173-179.
36. Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322(15):1028-1031.
37. Potter TG, Snider KR. Azithromycin for the treatment of gastroparesis. Ann Pharmacother. 2013;47:411-415.
To comment on this article, contact rdavidson@uspharmacist.com.





