US Pharm. 2024;49(1):HS7-HS12.
ABSTRACT: Traumatic brain injury (TBI) is a significant public-health problem affecting persons of all ages, with the youngest and oldest populations being the most vulnerable. Symptoms of moderate or severe TBI are likely to persist long-term, resulting in significant disability and affecting physical, cognitive, behavioral, and functional domains. Acute care management includes patient stabilization and prevention of secondary brain injury. Pharmacologic management of TBI includes reduction of intracranial pressure, coagulopathy, and neuropsychiatric care (seizure occurrence, cognitive disturbances). Fall-risk assessment and other educational programs are recommended in all settings. In acute and primary care settings, pharmacists may assist patients and providers with TBI management.
Traumatic brain injury (TBI) remains one of the leading causes of death and disability in the United States and is a significant public-health problem. The global prevalence of TBI is estimated at 50 million to 60 million, translating to 8.1 million years lived with a disability.1,2 According to the CDC, 190 TBI-related deaths take place each day in the U.S., and it was calculated that 2.5 million TBI-related emergency department (ED) visits, 282,000 hospitalizations, and 56,000 deaths occurred in 2013.3,4 The direct and indirect costs associated with TBI in the U.S. are significant: an estimated $76 billion or more across a lifetime.5 Research on 5-year outcomes of TBI in the U.S. found that 22% of patients died, 30% became worse, 22% remained the same, and 26% improved.5 Approximately 3.2 million to 5.3 million individuals in the U.S. are living with a TBI-related disability; however, these data do not include untreated TBI or TBI that is diagnosed in outpatient practices or the ED.6
The leading cause of TBI is a fall, with rates highest in the youngest (children aged 0-4 years) and oldest populations (adults aged >75 years).7 Most hospitalizations due to TBI are related to a fall, whereas motor-vehicle accidents account for most TBI-related deaths. Across all age groups, females have a lower rate of TBI occurrence than males.7 TBI related to sports and recreation accounted for 283,000 ED visits from 2010 to 2016, with 45% due to contact sports.8 Data indicate that from 2000 through 2011, a TBI diagnosis was made in 4.2% of service members in the Army, Navy, Air Force, and Marine Corps.6,9
The impact of TBI spans multiple domains, and ongoing research and education regarding prevention and management are needed.
Definition and Classification
TBI is most simply defined as a disruption to normal brain function that is caused by an external force.6,10,11 Multiple types of external force can result in TBI, including the head striking or being struck by an object; acceleration or deceleration of the brain without external impact (e.g., whiplash); a foreign body penetrating the brain (e.g., gunshot wound); or blast/explosion forces.10 Brain-function alteration may present with one or more of the following clinical signs6,10,11:
• Loss or decreased level of consciousness for any period
• Any memory loss of events immediately before or after injury (retrograde or posttraumatic amnesia)
• Neurologic deficits that may or may not be transient (e.g., weakness, balance/coordination loss, disruption of vision, changes in speech/language, and/or sensory loss)
• Mental-state alterations of any kind at the time of injury (e.g., confusion, disorientation, slowed thinking, concentration difficulties).
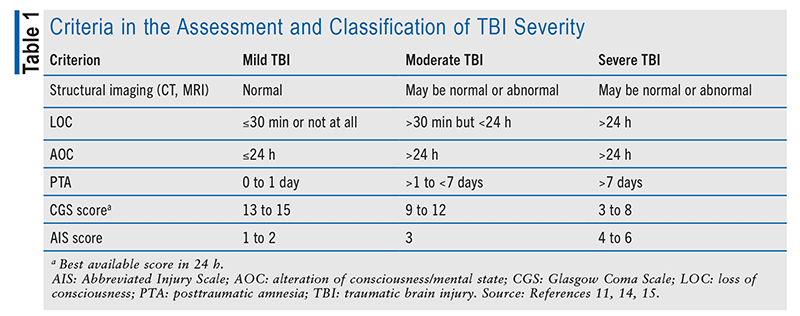
TBI is classified based on the severity of injury and the resulting presentation. One common method for determining TBI severity is the Glasgow Coma Scale (GCS), which was developed to assess the depth and duration of coma and impaired consciousness.6,11-13 The GCS evaluates motor responsiveness, verbal performance, and eye opening. Limitations to GCS use include mechanical sedation and paralysis, intubation, drug/alcohol intoxication, and organ failure.6 A GCS score of 13 to 15 denotes mild TBI, and moderate and severe TBI are indicated by GCS scores of 9 to 12 and <9, respectively.6,11,13 Imaging, duration of loss of consciousness, alteration of consciousness/mental state, posttraumatic amnesia (PTA), and the Abbreviated Injury Scale (AIS) are additional criteria used to classify TBI severity (TABLE 1).6,11,14,15
Pathophysiology
Focal injuries to the brain include hematomas (epidural, subdural), hemorrhages (subarachnoid, intraparenchymal), contusions, and fractures occurring in a specific location. Diffuse brain injury may also occur and is more widespread. One or both injury types may be experienced by the patient at the same time.6
The injury mechanism by which TBI transpires is neurometabolic in nature.16,17 Brain injury results in neuronal dysfunction due to axonal stretching and axonal injury. Ionic flux (potassium efflux, calcium/sodium influx) occurs in response to an abrupt release of excitatory neurotransmitters, namely glutamate. Voltage- or ligand-gated ion channels are then triggered, creating a spreading depression–like state and possibly accounting for post-TBI impairments. A significant increase in glucose metabolism causes an energy crisis, the result of adenosine phosphate-requiring ion pumps working overtime. A mismatch in energy supply and demand results from this increase in energy demand in the context of normal or reduced cerebral blood flow. Calcium is then sequestered into mitochondria due to a period of intracellular calcium flux, leading to mitochondrial dysfunction and worsening energy crisis. Free radicals produced during the altered intracellular redox state then cause oxidative stress. The collapse of neurofilament side-arms of neurons and glia compromises axonal structural integrity and has been linked to calcium flux.16,17
Management
The primary objectives of managing moderate and severe TBI are to stabilize the patient; maintain brain perfusion by preventing and treating hypoxia, hypotension, and increased intracranial pressure (ICP); and reduce the potential for secondary brain injury. It is critical to maintain adequate oxygenation (partial pressure of oxygen in arterial blood >60 mmHg), and endotracheal intubation may be required. Continuous monitoring of heart rate, blood pressure, blood oxygen level (via pulse oximetry), respiratory status, and temperature is recommended. The goal is to avoid hypotension, hyper- or hypoventilation, and hypoxia.12,18,19
To improve the patient’s outcome and reduce the in-hospital length of stay and 2-week postinjury mortality, monitoring of ICP is recommended.20 Cerebral perfusion pressure (CPP) is the difference between mean arterial pressure (MAP) and mean ICP. CPP will decrease if ICP increases. Brain ischemia may occur if CPP drops <50 mmHg, and brain death takes place if ICP increases to equal MAP.12,21 It is crucial to monitor and manage ICP. ICP >22 mmHg is associated with increased mortality; therefore, the treatment of ICP >22 mmHg is recommended.20
Mannitol and hypertonic saline are hyperosmolar agents used to lower ICP in severe TBI.12,21,22 Mannitol is administered IV at a dosage of 0.25 g/kg to 1 g/kg every 4 to 6 hours. Adverse effects of mannitol may include a rebound increase in ICP, electrolyte abnormalities, acute renal dysfunction, and diuresis. The use of mannitol should be avoided in patients with renal failure or congestive heart failure. Hypertonic saline in concentrations of 3% to 23.4% is an alternative to mannitol. Although guidelines do not recommend one agent over the other, proposed advantages of hypertonic saline use include a greater decrease in ICP, increase in CPP, volume repletion, increased brain oxygenation, and increased blood pressure. Potential adverse effects include prolonged coagulation times and decreased platelet aggregation, hypokalemia, and hyperchloremic acidosis.12,21,22 Additional methods for reducing ICP include sedation, analgesia, and temperature modulation.20
Nutrition, seizure prophylaxis, stress ulcer prophylaxis, infection prevention/control, glycemic control, blood pressure control, and venous thromboembolism prophylaxis are other important management strategies in the TBI patient; however, they are beyond the scope of this article. ICP management and neuropsychiatric symptoms of TBI are discussed elsewhere.22
Elderly Patients: One challenge to managing TBI in elderly patients is that many persons aged >65 years receive chronic anticoagulation therapy with warfarin or direct oral anticoagulants (DOACs). Individuals may also be receiving antiplatelet therapy with aspirin, clopidogrel, ticagrelor, or prasugrel alone or in conjunction with DOACs or warfarin. As an early management goal, the American College of Surgeons recommends anticoagulant reversal when feasible because anticoagulants and antiplatelets can exacerbate TBI sequelae.23
To determine the effect of preinjury anticoagulant use on outcomes in older adults with TBI, one study evaluated TBI patients aged >65 years who suffered a ground-level fall with an AIS head score >3.24 Compared with patients not on anticoagulants, there was an increased risk of mortality or hospice in the DOAC (odds ratio [OR] 1.67; 95% CI, 1.07-2.59) and warfarin groups (OR 1.60; 95% CI, 1.27-2.01). Additionally, the risk of mortality or hospice was greater with the use of warfarin plus antiplatelet therapy (OR 1.61; 95% CI, 1.18-2.21) compared with DOAC plus antiplatelet therapy (OR 0.93; 95% CI, 0.46-1.87). Patients with preinjury use of warfarin had significantly worse head injuries.24
A prospective observational trial involving 1,847 TBI patients (mean age 74.9 years) taking aspirin, clopidogrel, warfarin, and DOACs found that the DOAC group had less risk of intracranial hemorrhage on univariate (24% vs. 31%) or multivariate analysis (incidence rate ratio 0.78; 95% CI, 0.61-1.01; P = .05), whereas those taking aspirin were found to have the highest risk and highest rate.25 Other studies have identified an increased risk of adverse outcomes—specifically, mortality—in patients anticoagulated on warfarin compared with DOACs; however, further research is needed.26-29
Coagulopathy (either excessive bleeding or excessive clotting) may occur in TBI. In a meta-analysis, the reported overall prevalence of coagulopathy was 32.7%, and coagulopathy was related to unfavorable outcome (OR 36.3; 95% CI, 18.7-70.5) and mortality (OR 9.0; 95% CI, 7.3-11.6).29
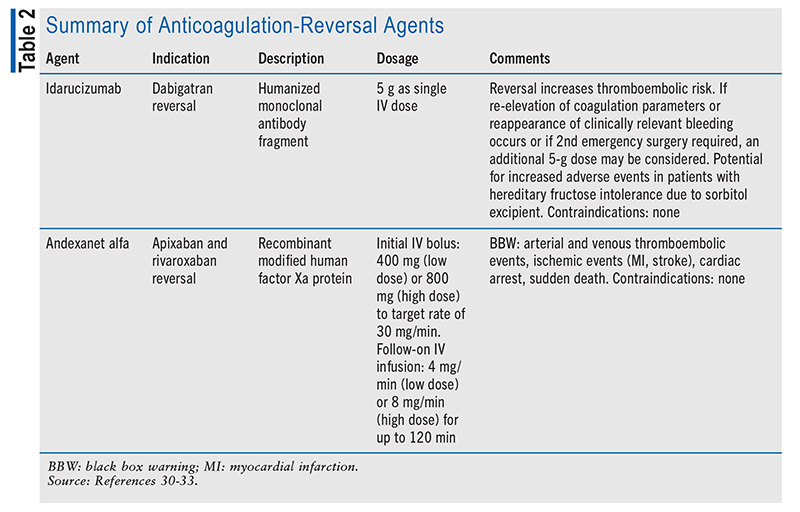
Warfarin-reversal agents include vitamin K, prothrombin complex concentrate, and fresh frozen plasma.30,31 Idarucizumab was approved in 2015 for the reversal of dabigatran. In 2018, andexanet alfa was approved for the reversal of apixaban and rivaroxaban. DOAC-reversal agents are summarized in TABLE 2.32,33 Limited data are available for the use of platelet transfusions to reverse antiplatelet therapies, and their use may worsen clinical outcomes.34,35
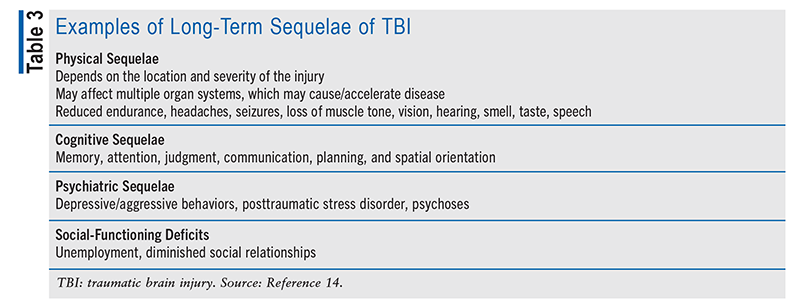
Postacute Care of TBI: Long-term disability occurs at a rate of 40% in patients hospitalized with moderate to severe TBI, owing to sustained impairments from the injury.14 TABLE 3 lists examples of long-term sequelae from TBI. Injury type and severity may be associated with specific impairments and can affect physical, cognitive, and behavioral domains. After acute treatment of TBI, medically stable patients may begin postacute care that includes many facets of rehabilitation. Regardless of the patient’s impairment, the goal of rehabilitation is to restore the patient to meaningful participation at home, at work, and in the community.14
Program delivery methods vary in frequency and type; they may be individual or group sessions, involve all-day or short daily treatments, and take place one or more days per week. No one method or program type has been shown to be superior.14 Most programs are multidisciplinary and focus on restoring the patient to meaningful functional status. Recommendations for individualizing rehabilitative needs include assessments of motor impairments; pain; speech/swallowing impairments; sensory dysfunction that may impact safety (vision, hearing, numbness); autonomic dysfunction; cognitive dysfunction (memory, communication); potential emotional/behavioral issues; and presence/absence of PTA. Assessments should be performed by a neuropsychologist, occupational therapist, and speech-language pathologist.36 Personal factors (e.g., cultural/educational background, language fluency) and any chronic preinjury health conditions should be evaluated. It is recommended to focus on real-world activities, train in compensatory strategies, educate/train caregivers and family, and incorporate functional adaptations.36
With regard to medication use, nonpharmacologic methods are recommended prior to use of pharmacologic methods when feasible. If pharmacologic management is initiated, goals include using the lowest effective dose, maximizing medication coverage to more than one symptom when possible, and minimizing side effects.36
Fall Prevention and Education: One in four individuals aged >65 years reports a fall each year, with 37% requiring medical attention for a sustained injury.37,38 Deaths from falls in older adults continue to increase. Healthcare-system costs for nonfatal falls in older adults are just over $49 billion annually.37,39 Given that ground-level falls account for a significant number of TBIs, especially in older patients, fall-prevention education and assessment of fall risk are necessary. Common tools for evaluating fall risk include STEADI (Stopping Elderly Accidents, Deaths, & Injuries), Tinetti mobility assessment, Timed Up and Go (TUG), Berg Balance Scale, Morse Scale, and Five Times Sit to Stand.40 Multiple-fall prevention and other educational resources are available from the CDC, including a compendium of fall interventions.41
The Pharmacist’s Role
In acute and primary care settings, pharmacists may assist patients and providers with TBI management. Numerous acute-care protocols focusing on various aspects of TBI management involve different levels of pharmacotherapy. Pharmacists can make medication recommendations to improve overall outcomes; they can also educate providers and the care team regarding pharmacologic options for acute management and long-term sequelae. Pre- and postinjury medication profiles should be assessed, with the goals of optimizing drug selection, minimizing side effects, using the lowest effective dose necessary for symptom management, and reducing drug-drug interactions. Pharmacists in all settings may make recommendations for medication management of acute and chronic health conditions to limit the use of medications that may worsen physical and cognitive symptoms of TBI.
REFERENCES
1. GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56-87.
2. Maas AI, Menon DK, Manley GT, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21(11):1004-1060.
3. CDC. Traumatic brain injury & concussion. www.cdc.gov/traumaticbraininjury/index.html. Accessed November 12, 2023.
4. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16.
5. CDC. Moderate and severe TBI. www.cdc.gov/traumaticbraininjury/moderate-severe/index.html. Accessed November 12, 2023.
6. National Center for Injury Prevention and Control. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. Atlanta, GA: CDC; 2015.
7. Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta, GA: CDC, National Center for Injury Prevention and Control; 2010.
8. Waltzman D, Womack LS, Thomas KE, Sarmiento K. Trends in emergency department visits for contact sports-related traumatic brain injuries among children—United States, 2001-2018. MMWR Morb Mortal Wkly Rep. 2020;69(27):870-874.
9. CDC, National Institutes of Health, Department of Defense, and Department of Veterans Affairs Leadership Panel. Report to Congress on traumatic brain injury in the United States: understanding the public health problem among current and former military personnel. June 2013. www.cdc.gov/traumaticbraininjury/pdf/report_to_congress_on_traumatic_brain_injury_2013-a.pdf. Accessed December 6, 2023.
10. Menon DK, Schwab K, Wright DW, et al. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637-1640.
11. Department of Veterans Affairs; Department of Defense. VA/DoD clinical practice guideline for the management of concussion-mild traumatic brain injury. Version 2.0–2016. www.va.gov/covidtraining/docs/mTBICPGFullCPG50821816.pdf. Accessed November 7, 2023.
12. Boucher BA, Wood C. Acute management of the brain injury patient. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill Education; 2014.
13. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84.
14. Brasure M, Lamberty GJ, Sayer NA, et al. Multidisciplinary Postacute Rehabilitation for Moderate to Severe Traumatic Brain Injury in Adults. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
15. Orman JAL, Kraus JF, Zaloshnja E, et al. Epidemiology. In: Silver JM, McAllister TW, Yudofsky SC, eds. Textbook of Traumatic Brain Injury. 2nd ed. Washington, DC: American Psychiatric Publishing, Inc; 2011:3-22.
16. Giza GC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228-235.
17. Giza GC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):s24-s33.
18. Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):s1-s106.
19. Rajajee V. Management of acute moderate and severe traumatic brain injury. UpToDate. Waltham, MA: UpToDate Inc. www.uptodate.com. Accessed December 6, 2023.
20. Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6-15.
21. Kassel L, Salvatore D. CNS hemorrhages. In: PSAP 2018: Neurology and Psychiatry. Lenexa, KS: American College of Clinical Pharmacy; 2018:47-75.
22. Woodard TJ, Hill AM, Bell-Lynum KS. Managing neuropsychiatric symptoms in traumatic brain injury. US Pharm. 2015;40(11):HS3-HS9.
23. American College of Surgeons Trauma Quality Improvement Program. Best practices in the management of traumatic brain injury. January 2015. www.facs.org/media/mkej5u3b/tbi_guidelines.pdf. Accessed November 12, 2023.
24. Hecht JP, LaDuke ZJ, Cain-Nielsen AH, et al. Effect of preinjury oral anticoagulants on outcomes following traumatic brain injury from falls in older adults. Pharmacotherapy. 2020;40(7):604-613.
25. Kobayashi L, Barmparas G, Bosarge P, et al. Novel oral anticoagulants and trauma: the results of a prospective American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2017;82(5):827-835.
26. Maung AA, Bhattacharya B, Schuster KM, Davis KA. Trauma patients on new oral anticoagulation agents have lower mortality than those on warfarin. J Trauma Acute Care Surg. 2016;81(4):652-657.
27. Feeney JM, Neulander M, DiFiori M, et al. Direct oral anticoagulants compared with warfarin in patients with severe blunt trauma. Injury. 2017;48(1):47-50.
28. Prexl O, Bruckbauer M, Voelckel W, et al. The impact of direct oral anticoagulants in traumatic brain injury patients greater than 60-years-old. Scand J Trauma Resusc Emerg Med. 2018;26(1):20.
29. Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien). 2008;150(2):165-175.
30. Vitamin K1 (phytonadione injection, emulsion) product information. Lake Forest, IL: Hospira, Inc; April 2021.
31. Kcentra (prothrombin complex concentrate [human]) product information. Kankakee, IL: CSL Behring LLC; May 2023.
32. Praxbind (idarucizumab) product information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; October 2021.
33. Andexxa (coagulation factor Xa [recombinant], inactivated-zhzo) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; February 2023.
34. Joseph B, Pandit V, Meyer D, et al. The significance of platelet count in traumatic brain injury patients on antiplatelet therapy. J Trauma Acute Care Surg. 2014;77(3):417-421.
35. Nishijima DK, Zehtabchi S, Berrong J, Legome E. Utility of platelet transfusion in adult patients with traumatic intracranial hemorrhage and preinjury antiplatelet use: a systematic review. J Trauma Acute Care Surg. 2012;72(6):1658-1663.
36. Bayley MT, Janzen S, Harnett A, et al. INCOG 2.0 guidelines for cognitive rehabilitation following traumatic brain injury: methods, overview, and principles. J Head Trauma Rehabil. 2023;38(1):7-23.
37. CDC. Older adult falls data. www.cdc.gov/falls/data/index.html. Accessed November 12, 2023.
38. Kakara R, Bergen G, Burns E, Stevens M. Nonfatal and fatal falls among adults aged >65 years—United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2023;72(35):938-943.
39. Florence CS, Bergen G, Atherly A, et al. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693-698.
40. Strini V, Schiavolin R, Prendin A. Fall risk assessment scales: a systematic literature review. Nurs Rep. 2021;11(2):430-443.
41. Burns E, Kakara R, Moreland B. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 4th ed. Atlanta, GA: CDC, National Center for Injury Prevention and Control; 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






