US Pharm. 2018;43(4):HS-7-HS-12.
ABSTRACT: Antibiotic-resistant organisms account for more than 2 million infections and 23,000 deaths annually in the United States.1 The overuse of antibiotics is believed by many to have led to the development of antibiotic resistance. Although the development of antimicrobial-stewardship programs in hospital and acute-care settings has led to a decrease in morbidity, mortality, and overuse of antibiotics, rates of antibiotic resistance continue to rise. This failure to curb resistance rates is a multifactorial problem involving many aspects of the healthcare team and acute-care setting, and it must be addressed.
Antibiotics are one of the most revolutionary discoveries to impact global health. However, the overuse and misuse of these agents has led to parallel changes in both public health and clinical practice. Recent reports by the CDC indicate that 30% to 50% of antibiotic use in hospitals is unnecessary or unwarranted.2 Furthermore, the burgeoning effects of antibiotic misuse have led to significant antibiotic-resistance issues that pose a public-health risk to both infected and noninfected patients.3
History of Antimicrobial Stewardship
Described as early as the 1940s by Alexander Fleming, who lamented the waning efficacy of penicillin due to its overuse, the concept of antibiotic stewardship is not new.4 The growing threat of antibiotic resistance had led infectious-disease professional organizations to provide support and guidelines to combat the growing threat.5 In 2009, the CDC launched the first educational effort to promote improved use of antibiotics in acute-care hospitals, and in 2013, the agency highlighted the need to improve antibiotic use as one of four key strategies required to address the problem of antibiotic resistance in the U.S.6,7 In addition, accrediting agencies such as the Joint Commission and government-based organizations, including the Centers for Medicare & Medicaid Services (CMS) have come forward to emphasize the need for and importance of antibiotic stewardship programs (ASPs) across a variety of clinical practice areas. In January 2017, these efforts culminated in recommendations by the Joint Commission calling for all hospitals to have stewardship programs.8 In addition, CMS proposed a rule change that would require all hospitals in the United States to implement an ASP.8
Core Elements of Antibiotic Stewardship
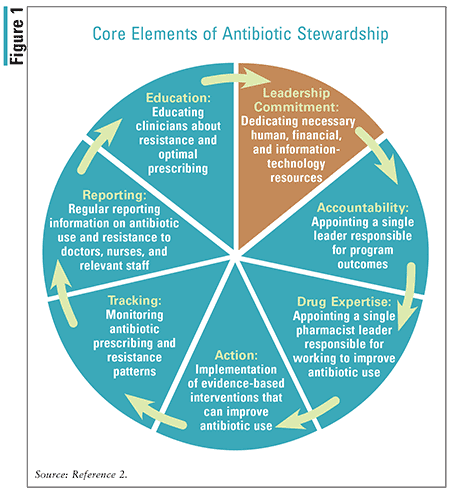
The CDC has long recognized the growing threat of antibiotic-resistant organisms and the importance of employing antibiotic stewardship to combat antibiotic-related adverse events. Antibiotic stewardship is defined by the CDC as set of commitments and actions designed to “optimize the treatment of infections while reducing the adverse events associated with antibiotic use.”2 Therefore, any healthcare setting in which antibiotics are used should institute antibiotic-stewardship practices aimed at improving antibiotic-related patient safety and slowing the spread of antibiotic resistance.2 Figure 1 summarizes the core elements of successful hospital ASPs.2
Role of the ASP in Limiting Antibiotic Resistance
Although the epidemiology of antibiotic resistance is complex, antibiotic overuse has been recognized as a major driver for the emergence and dissemination of resistant bacteria. The incidence of overuse may occur in multiple practice areas. For example, the clinical practitioner may provide additional doses of antibiotics, although not indicated, to potentially minimize the patient’s risk of developing a surgical-site infection, or empirically treat an infection in a hospitalized patient while diagnostic information is being obtained, but fail to revisit the selection of the antibiotic after more clinical and laboratory data becomes available. In the outpatient setting alone, of the estimated 154 million prescriptions for antibiotics written in doctors’ offices and emergency departments each year, 30% are unnecessary.9 Each of these scenarios poses significant risk and potentially contributes to the growing threat of antibiotic resistance.
Some research studies have demonstrated the beneficial impact antimicrobial stewardship-based initiatives can have on antimicrobial resistance.10,11 Elligsen and colleagues demonstrated that by conducting a prospective audit of all critical-care patients on their third or tenth day of broad-spectrum antibiotic therapy and communicating suggestions for antimicrobial optimization to the critical care team, practitioners could have a potentially positive impact on antimicrobial prescription patterns in the intensive care unit (ICU) and reduce the emergence of resistance.10 A prospective study performed in the ICU of a public hospital confirmed that audit and feedback, through the use of a critical-care pharmacist and/or infectious-disease physician, were independently associated with appropriate antimicrobial selection and prevention of resistance.11
The Reality of Antibiotic Stewardship
A survey taken in 2016 reported that only 64.2% of hospitals nationwide had met all core elements of a hospital ASP.12 While this figure will likely change, particularly because of the inclusion of antimicrobial stewardship standards in Joint Commission requirements, it is expected to fall short of meeting the 2020 national goal of 100% of hospitals taking part in an ASP.8,12 Various clinical-practice settings face similar challenges in implementing and sustaining an ASP.
In the 1970s, evolutionary changes in medicine led to a perception that the specialty of infectious diseases (ID) might become extinct; some even declared that there was minimal need for ID experts.13,14 Today, in many instances, there is a lack of trained ID physicians and pharmacists allocated to ASP leadership roles, diminishing the ability of ASPs to mature and develop into integral parts of a hospital’s workflow.13 In addition, the challenge of allocating proper funding and resources to ASPs often leads to programs in which essential components and team members may be lacking. Finally, the lack of a strong, integrated information technology network system to properly monitor, track, and analyze antibiotic use across a wide range of settings in hospitals and acute-care settings has prevented the full integration of ASPs into hospital networks.1
Innovative Antibiotic Stewardship Solutions Are Key
Antibiotic stewardship calls for a broad array of initiatives aimed at ensuring the right antibiotic is given to the right patient for the right amount of time. As such, the specific initiatives that hospitals choose to implement are meant to integrate into the larger hospital workflow. Research has recognized that there is no single prototype for a program to optimize antibiotic prescribing in hospitals. The complexity of medical decision-making surrounding antibiotic use and the variation in the size of U.S. hospitals and types of care provided require flexibility in implementation.16,17
The CDC classifies antibiotic-stewardship initiatives into three categories: broad, pharmacy-driven, and infection- and syndrome-specific. Hospitals are advised to implement the programs that are best suited to them, based on available resources, local needs, and national guidelines. The implementation of too many initiatives at once may complicate operations, so a tailored approach to each institution may be warranted.2 Although the lack of resources in some hospitals precludes the hiring of full-time ID specialists, whether they are pharmacists or physicians, innovative solutions exist. For example, the decentralization of ID experts across a number of hospitals, including part-time or off-site experts, allows hospitals to take part in ASP programs without going over budget. Strong-performing ASPs also exhibit more autonomy and flexibility, often existing as an independent entity from infection-control programs. The involvement of leadership, such as chief medical officers, pharmacy directors, and nursing leaders, may boost both the morale and confidence of those implementing ASPs, as well as involve more healthcare workers in taking part in the program. Ideally, physicians, nurses, microbiologists, pharmacists, and other hospital personnel would actively participate in stewardship programs.
Kapadia and colleagues used qualitative methods to identify and describe characteristics of leading ASPs and novel strategies for stewardship.1 They concluded that ASPs that have demonstrated success encompass an evolving role for the ASP that is no longer focused on a “top-down” methodology but rather empowers physicians and pharmacists across disciplines to champion antibiotic stewardship initiatives.1 Figure 2 summarizes the key elements of successful ASPs.
Conclusion: A Team Approach
Meeting the CDC’s 2020 goal of all hospitals implementing an ASP does not entirely solve the problem of antibiotic resistance or the poor use of antibiotics. A thorough understanding of the needs of a hospital, as well as the human and financial resources available, is necessary. Public understanding of the issues related to inappropriate antimicrobial use is important for tackling antimicrobial resistance. In addition, there is a need for a comprehensive understanding among all stakeholders within each clinical-practice setting that all must be good stewards in the use of antibiotics.
Pharmacists are expected to respond to antibiotic-therapy situations that may call for changes from IV to oral therapy, dose adjustments, and dose optimization; they will also be called upon to identify unnecessary duplicate therapy and drug interactions, and clarify when an antibiotic should be initiated and discontinued. However, any healthcare professional who interacts with antibiotics or any patient who receives antimicrobial therapy can have an impact on their use. Prescribers of antibiotics should be fully engaged in and supportive of efforts to improve antibiotic use in hospitals. Nurses can assure that cultures are performed before starting antibiotics, review medications, and encourage discussions of antibiotic treatment, indication, and duration.2 Antibiotic stewardship must be a coordinated, multidisciplinary, interprofessional team approach in order to be successful.
REFERENCES
1. Kapadia SN, Abramson EL, Carter EJ, et al. The expanding role of antimicrobial stewardship programs in hospitals in the United States: lessons learned from a multisite qualitative study. Jt Comm J Qual Patient Saf, 2018;44(2):68-74.
2. CDC. Antibiotic prescribing and use in hospitals and long-term care. Core elements of hospital antibiotic stewardship programs. February 23, 2017. www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html#_ENREF_57. Accessed January 21, 2018.
3. World Health Organization. Antimicrobial resistance. Updated April 2015. www.who.int/mediacentre/ factsheets/fs194/en/#. Accessed January 21, 2018.
4. Penicillin’s finder assays its future: Sir Alexander Fleming says improved dosage method is needed to extend use other scientists praised self-medication decried. The New York Times. June 26, 1945.
5. Shlaes DM, Gerding DM, John JF, et al. Guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997;18:275-291.
6. CDC. Antibiotic prescribing and use in hospitals and long-term care. www.cdc.gov/antibiotic-use/healthcare/. Accessed February 24, 2014.
7. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Systematic Reviews. 2013;4.
8. Joint Commission. New antimicrobial stewardship standard. Jt Comm Perspect. 2016;36(7):1-8.
9. CDC. CDC: 1 in 3 antibiotic prescriptions unnecessary. May 3, 2016. www.cdc.gov/media/releases/2016/p0503-unnecessary-prescriptions.html. Accessed January 18, 2018.
10. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361.
11. DiazGranados CA. Prospective audit for antimicrobial stewardship in intensive care: impact on resistance and clinical outcomes. Am J Infect Control. 2012;40(6):526-529.
12. CDC. Antibiotic prescribing and use in hospitals and long-term care: percent of hospitals with antibiotic stewardship programs by state, 2016. November 27, 2017. www.cdc.gov/antibiotic-use/community/images/materials/2016-Percentages-B.jpg. Accessed January 18, 2018.
13. Walensky RP, Del Rio C, Armstrong WS. Charting the future of infectious disease: anticipating and addressing the supply and demand mismatch. Clin Infect Dis. 2017;15;64:1299-1301.
14. Petersdorf RG. Whither infectious diseases? Memories, manpower, and money. J Infect Dis. 1986;153(2):189-195.
15. American Society of Health-System Pharmacists. Implementing antimicrobial stewardship programs in health systems: an interprofessional team approach. 2013; www.ashpadvantagemedia.com/downloads/2013-asp-discussion-guide.pdf. Accessed March 8, 2018.
16. Yam P, Fales D, Jemison J, et al. Implementation of an antimicrobial stewardship program in a rural hospital. Am Journal Health Syst Pharm. 2012;69(13):1142-1148.
17, Goff DA, Bauer KA, Reed EE, et al. Is the “low hanging fruit” worth picking for antimicrobial stewardship programs? Clin Infect Dis. 2012;55:587-592.
18. Kullar R, Golf DA, Schultz LT, et al. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin. Infect. Dis. 2013;57:1005-1013.
19. Box MJ, Sullivan EL, et al. Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy. 2015;35(3):269-276.
20. Kuper K. What makes a successful antibiotic stewardship program? Drug Topics. November 10, 2017. drugtopics.modernmedicine.com/drug-topics/news/what-makes-successful-antibiotic-stewardship-program. Accessed March 8, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.