US Pharm. 2023;48(11):32-36.
Gastric cancer, a form of cancer that originates in the stomach, results from uncontrolled growth of stomach cells. Unlike some malignancies, gastric cancer progresses gradually over an extended period. More than 90% of gastric cancers are adenocarcinomas, originating in the mucus-producing cells lining the innermost layer of the stomach. The remaining cases are mostly gastrointestinal neuroendocrine tumors, stromal tumors, and primary gastric lymphomas. Gastric adenocarcinomas can be classified into two main types: intestinal and diffuse. Intestinal gastric cancer is characterized by its gland-forming cohesive cells, and it also has well-differentiated cells, resembling normal cells. These cancers typically develop in the lower part of the stomach and often have an extended precancerous phase. On the other hand, diffuse gastric cancers are characterized by their noncohesive cells and poorly differentiated appearance. They tend to occur more frequently in the cardia region of the stomach.1,2
Despite a decline in its occurrence and mortality rates in recent decades, stomach cancer remains a significant global health challenge. In 2020, it ranked as the fourth most common cause of cancer-related deaths worldwide, following lung, colon, and liver cancer. During the early 20th century, gastric cancer was a leading cause of death from malignant tumors in the United States. However, in recent years, there has been a substantial reduction in both the incidence and fatality rates associated with stomach cancer. Nevertheless, it is estimated that in 2023 stomach cancer will claim approximately 11,130 lives in the U.S., constituting 1.8% of all cancer-related deaths, with 26,500 new cases reported accounting for around 1.5% of all new cancer diagnoses. The 5-year survival rate for localized stomach cancers remains 75%, but this rate drops to 35% for regional stomach cancers. Metastatic stomach cancers have the lowest 5-year survival rate: only 7%. Individuals aged 65 to 74 years are the most frequently diagnosed demographic for gastric cancers. Although stomach cancer’s prevalence has been declining over the years, it is essential for individuals to remain vigilant and undergo regular screenings, especially if they have risk factors such as a family history of gastric cancer or a history of certain infections, as early detection can significantly improve outcomes.3-5
Risk Factors
Gastric cancer is influenced by a multitude of risk factors that encompass dietary habits, lifestyle choices, genetic predispositions, and various environmental factors. High salt intake, inadequate consumption of fresh fruits and vegetables, and the excessive consumption of red and processed meats have been linked to an elevated risk of gastric cancer. Lifestyle factors such as smoking and heavy alcohol consumption contribute significantly to this risk. Smoking had a significant impact on increasing the risk of this cancer. Alcohol consumption was associated with an elevated risk, especially in cases of heavy drinking. Genetic factors, including specific gene polymorphisms and family history, play a role in an individual’s susceptibility to gastric cancer. The prevalence of gastric cancers is notably influenced by the presence of certain tumor-suppressor genes, including the mutated in colorectal cancer (MCC) gene, the adenomatous polyposis coli (APC) gene, and the tumor-suppressor gene p53. Additionally, a hereditary mutation in the CDH1 allele has been identified as a correlated factor in the development of gastric cancers. Demographic characteristics such as age and gender impact risk, with older individuals and men being more vulnerable. Socioeconomic status, education level, and occupational exposures are additional risk factors that underscore the complexity of this disease.6-8
Infections such as Helicobacter pylori and possible viral infections can elevate the risk of developing gastric cancer. H pylori, a gram-negative, microaerophilic, spiral bacterium typically residing in the stomach, is responsible for this increased risk. It targets the stomach lining and interferes with the enzyme urease, reducing the acidity of stomach acid. Many individuals carry this bacterium in their stomach without experiencing symptoms. In the early stages, the infection triggers inflammation, subsequently progressing to mucosal atrophy, metaplasia, dysplasia, and ultimately the development of carcinoma.6-8
The utilization of proton pump inhibitors (PPIs) has sparked interest regarding their potential connection to gastric cancer risk. Despite PPIs being generally regarded as safe and efficacious, concerns have arisen over the prolonged use of PPIs and their possible link to an elevated risk of gastric cancer. A population-based cohort study revealed that individuals who initiated PPI treatment had a 45% higher likelihood of developing gastric cancer compared with those commencing treatment with H2 receptor antagonists (H2RAs). Moreover, a meta-analysis indicated that PPI users faced an 80% elevated risk of gastric cancer relative to nonusers. Notably, extended PPI usage exceeding 3 years was associated with an increased risk. The association between PPI use and gastric cancer risk is biologically plausible and can be attributed to various factors. PPIs can lead to heightened gastrin levels due to reduced gastric acidity, and gastrin is known to stimulate cell growth, potentially leading to hyperplasia. Prolonged PPI usage may also modify the gut microbiome, reducing microbial diversity, which has been linked to an increased risk of gastric cancer. Furthermore, persistent acid suppression by PPIs may be linked to atrophic gastritis, a precursor to gastric cancer, although this link remains subject to dispute. In contrast, H2RAs, which exert a milder acid-suppressing effect by blocking histamine, are less likely to induce hypergastrinemia and theoretically pose a lower risk of gastric cancer compared to PPIs.9,10
Screening and Diagnosis
In the U.S., screening and diagnosis of gastric cancer typically involve a combination of methods aimed at early detection and accurate evaluation. However, it is important to note that gastric cancer screening is not as widely practiced as for some other cancers, such as breast or colon cancer, due to its relatively lower prevalence in the U.S. population. Currently, there are no screening guidelines for gastric cancer; however, screening should be considered in individuals who are considered high risk, including immigrants from regions associated with a high risk of gastric cancer (East Asia, Russia, or South Africa) or those with a family history or specific genetic predispositions. During endoscopy, a flexible tube with a camera is inserted through the mouth to visualize the stomach lining and take tissue samples (biopsies) for examination. Diagnosis of gastric cancer is confirmed through histopathology, in which pathologists analyze the biopsied tissue samples for cancerous cells. Other diagnostic tools (i.e., imaging studies such as CT scans and MRI) may be employed to assess the extent of cancer and evaluate potential metastasis.
Early diagnosis is crucial for better treatment outcomes, and individuals with symptoms such as persistent abdominal pain, unexplained weight loss, or difficulty swallowing should seek medical evaluation promptly. It is essential for healthcare providers and patients to collaborate in identifying and addressing potential risk factors and symptoms associated with gastric cancer to ensure timely diagnosis and treatment.1,11-15
Treatment
Gastric cancer, known for its challenging nature, necessitates a multifaceted approach to treatment. The primary objectives of therapy are to eradicate the cancer, if possible; extend survival rates; enhance the patient’s quality of life; and prevent complications. Treatment options for gastric cancer encompass a range of strategies, including surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy.16
Surgical interventions include endoscopic mucosal resection, gastrectomy (subtotal or total), endoluminal stent placement, endoluminal laser therapy, and gastrojejunostomy. Endoscopic mucosal resection is a minimally invasive technique for early lesions, while gastrectomy involves the partial or total removal of the stomach. Procedures such as stent placement and laser therapy address blockages in the stomach. Gastrojejunostomy is usually reserved for palliative care.17-19
Radiotherapy, often used in conjunction with surgery or chemotherapy, uses radiation beams (photons, protons, or electrons) to target localized cancers. Proton beams are more precise, minimizing damage to healthy tissue. Common side effects include skin issues, nausea, fatigue, and difficulty eating.20
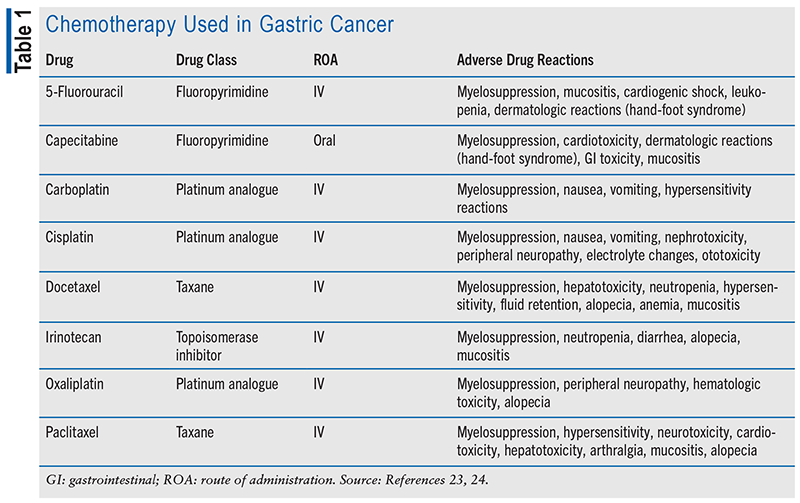
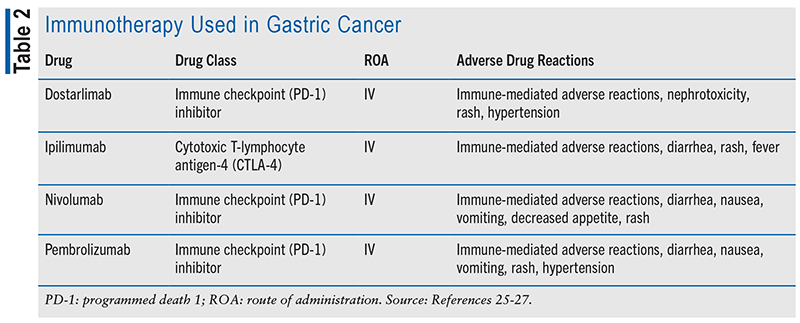
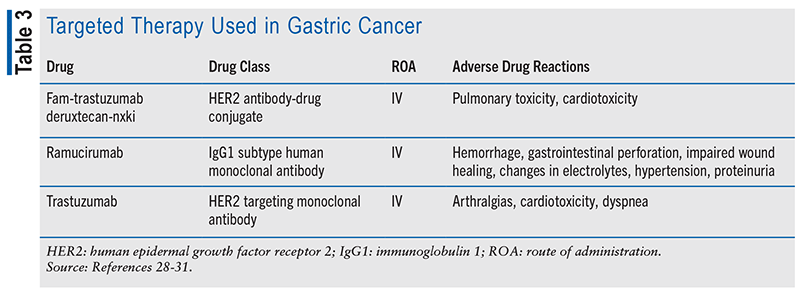
Chemotherapy is a mainstay in gastric cancer treatment, featuring drugs such as capecitabine, cisplatin, and paclitaxel (see TABLE 1). Various combination regimens are employed based on disease stage. Immunotherapy has recently become crucial, with immune checkpoint inhibitors such as pembrolizumab and nivolumab approved for advanced or metastatic gastric cancer. These agents boost the immune system’s ability to recognize and attack cancer cells when used alongside chemotherapy (see TABLE 2). Targeted therapy is another significant advancement (see TABLE 3). Drugs such as trastuzumab and ramucirumab target specific molecules in cancer cells to impede their growth and survival. Immunotherapy and targeted drug therapies hold promise for improving survival rates and reducing side effects compared with traditional chemotherapy but do not provide a cure. Overall, the evolving landscape of gastric cancer treatment offers hope for better outcomes and improved quality of life for patients.13,14,21
Role of the Pharmacist
Pharmacists play a pivotal role in providing essential support to individuals facing gastric cancer. With their profound knowledge of pharmaceuticals, pharmacists are well equipped to oversee and optimize the medication regimens of gastric cancer patients. Their expertise contributes to improving treatment effectiveness while minimizing any adverse effects. Additionally, pharmacists actively monitor patients for potential adverse events associated with their treatments and collaborate closely with healthcare teams to manage these issues effectively. This includes a thorough assessment of vital laboratory values, such as blood counts and liver function, to ensure the safety of patients throughout their cancer treatment journey.22
Moreover, pharmacists are instrumental in providing patient education, a practice consistently linked to higher patient-satisfaction rates and improved learning outcomes. Effective patient education also correlates with increased medication adherence and better overall disease-related outcomes. Pharmacists offer comprehensive counseling to individuals with gastric cancer, assisting them in understanding their condition, exploring treatment alternatives, and comprehending potential side effects. They are readily available to address any concerns patients may have, offering invaluable emotional support and promoting adherence to medications, ultimately enhancing the prospects of successful treatment outcomes. In essence, pharmacists serve as indispensable members of the healthcare team for gastric cancer patients, leveraging their expertise in medication management, symptom alleviation, and patient education to elevate the standard of care and bolster the overall well-being of those grappling with this challenging disease.22
Conclusion
Gastric cancer continues to be a complex disease that requires a comprehensive strategy of understanding risk factors, emphasizing the importance of early detection, and gaining insight into the various therapeutic options. Ongoing research and the advancement of treatment modalities offer hope for improving outcomes and decreasing the burden of gastric cancer for patients around the world.

What Is Gastric Cancer?
Gastric cancer, also known as stomach cancer, is a condition that begins in the stomach’s inner lining when cells start growing uncontrollably. The most common type of stomach cancer is adenocarcinoma.
What Are the Risk Factors Associated With Stomach Cancer?
There are several known risk factors for stomach cancer. These include a diet high in smoked, salted, or pickled foods, smoking, and heavy alcohol consumption. A family history of stomach cancer also increases your risk. About 3% of stomach cancers are hereditary. There are also some genetic mutations that may increase your risk.
An important risk factor for developing stomach cancer is infection with Helicobacter pylori. H pylori is a type of bacterium that lives in the lining of the stomach and is spread through contaminated food and water. Infection with H pylori does not cause problems for most people, but in some, it can cause inflammation and stomach ulcers, which can lead to cancer.
What Are the Symptoms of Stomach Cancer?
There are often no symptoms of stomach cancer, particularly in the early stages. If there are symptoms, they may include problems swallowing, indigestion, vomiting, weight loss, feeling weak, feeling full after eating a small amount of food, and anemia. You should see a healthcare professional if you experience any of these symptoms. However, it is important to remember that these symptoms can also occur in people who do not have stomach cancer. They may be caused by other conditions.
How Is Stomach Cancer Diagnosed?
A diagnosis of stomach cancer is based on the results of examinations and tests. This may include endoscopy, a procedure that uses a thin tube with a camera to examine the stomach lining. During this time, a sample of any areas that look abnormal can be taken (biopsy) and collected for laboratory analysis. Your provider may also request CT scans and other imaging tests, along with blood work to check for markers that may indicate the presence of cancer.
How Is Stomach Cancer Treated?
Your treatment will depend upon the size, location, and stage of your tumor, as well as your general health and level of fitness. Options include surgery, chemotherapy, radiation therapy, target therapy, and immunotherapy.
What Can I Do to Prevent Stomach Cancer?
While some risk factors are beyond your control, you can take steps to reduce your risk. Maintain a healthy diet rich in fruits and vegetables. Limit your consumption of processed and high-sodium foods. If you are a smoker, consider quitting, and limit your alcohol intake.
REFERENCES
1. Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333(1):32-41.
2. National Cancer Institute. What is stomach cancer? www.cancer.gov/types/stomach. Accessed September 29, 2023.
3. National Cancer Institute. Cancer stat facts: stomach cancer. www.seer.cancer.gov/statfacts/html/stomach.html. Accessed September 29, 2023.
4. National Cancer Institute. Stomach cancer survival rates and prognosis. May 31, 2023. www.cancer.gov/types/stomach/survival. Accessed September 29, 2023.
5. Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. 2022;28(12):1187-1203.
6. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-713.
7. Hui Y, Tu C, Liu D, et al. Risk factors for gastric cancer: a comprehensive analysis of observational studies. Front Public Health. 2023;10:892468.
8. Zali H, Rezaei-Tavirani M, Azodi M. Gastric cancer: prevention, risk factors and treatment. Gastroenterol Hepatol Bed Bench. 2011;4(4):175-185.
9. Abrahami D, McDonald EG, Schnitzer ME, et al. Proton pump inhibitors and risk of gastric cancer: population-based cohort study. Gut. 2022;71(1):16-24.
10. Poly TN, Lin MC, Syed-Abdul S, et al. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers. 2022;14(13):3052.
11. Liu X, Cai H, Wang Y. Prognostic significance of tumor markers in T4a gastric cancer. World J Surg Oncol. 2012;10(1):68.
12. Matsuoka T, Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J Gastroenterol. 2018;24(26):2818-2832.
13. Wöll E, Eisterer W, Gerger A, et al. Treatment algorithm for patients with gastric adenocarcinoma: an Austrian consensus on systemic therapy. Anticancer Res. 2019;39(9):4589-4596.
14. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2022;20(2):167-192.
15. Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84(1):18-28.
16. Markman M. Realistic goals of cancer therapy: effective and humane care. Cleve Clin J Med. 1994;61(6):468-472.
17. Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635-1649.
18. ASGE Technology Committee; Hwang JH, Konda V, et al. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82(2):215-226.
19. Rodríguez JI, Kutscher M, Lemus M, et al. Palliative gastrojejunostomy in unresectable cancer and gastric outlet obstruction: a retrospective cohort study. Ann R Coll Surg Engl. 2021;103(3):197-202.
20. National Cancer Institute. External beam radiation therapy for cancer. May 1, 2018. www.cancer.gov/about-cancer/treatment/types/radiation-therapy/external-beam. Accessed September 29, 2023.
21. Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39(4):1179-1203.
22. Holle LM, Segal EM, Jeffers KD. The expanding role of the oncology pharmacist. Pharm (Basel). 2020;8(3):130.
23. Anand U, Dey A, Chandel AKS, et al. Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022;10(4):1367-1401.
24. Amjad MT, Chidharla A, Kasi A. Cancer chemotherapy. In: StatPearls. StatPearls Publishing; 2023. www.ncbi.nlm.nih.gov/books/NBK564367/. Accessed September 29, 2023.
25. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
26. Jaber N. Study details long-term side effects of immune checkpoint inhibitors. April 30, 2021. www.cancer.gov/news-events/cancer-currents-blog/2021/immune-checkpoint-inhibitors-melanoma-long-term-side-effects. Accessed September 29, 2023.
27. National Library of Medicine. JEMPERLI- dostarlimab injection. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=095eab9f-545a-4f12-bfb7-19477fb901a5. Accessed October 3, 2023.
28. Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16(1):57.
29. Singh AD, Parmar S. Ramucirumab (cyramza): a breakthrough treatment for gastric cancer. P T. 2015;40(7):430-468.
30. Greenblatt K, Khaddour K. Trastuzumab. In: StatPearls. StatPearls Publishing; 2023. www.ncbi.nlm.nih.gov/books/NBK532246/. Accessed October 3, 2023.
31. National Library of Medicine. ENHERTU- fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7e67e73e-ddf4-4e4d-8b50-09d7514910b6. Accessed October 3, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





