US Pharm. 2024;49(2):38-44.
ABSTRACT: Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by thrombosis or pregnancy morbidity coupled with persistent antiphospholipid antibodies. The antibodies contributing to APS diagnosis are lupus anticoagulant, anticardiolipin antibodies, and anti–beta-2 glycoprotein I antibodies. In 2023, the American College of Rheumatology and the European Alliance of Associations for Rheumatology jointly published updated classification criteria for APS. Management involves identifying and mitigating thrombosis risk factors. Vitamin K antagonists are the standard of care; the use of aspirin is controversial and depends on the patient’s risk profile. Direct oral anticoagulant use is generally not recommended, based on limited evidence. More research is needed to establish therapeutic recommendations for APS.
Antiphospholipid syndrome (APS) is a clinical autoimmune syndrome characterized by venous or arterial thrombosis and/or pregnancy morbidity coupled with persistent laboratory evidence of antiphospholipid antibodies (aPLs).1 It may be associated with other systemic autoimmune diseases, such as systemic lupus erythematosus (SLE), but it can also occur alone as primary APS. APS may be further characterized according to the type of clinical manifestation (thrombotic or obstetric), but both may be present in some cases.2 The term thrombotic APS describes APS involving the presence of venous, arterial, or microvascular thrombosis. Obstetric APS is defined as APS characterized by recurrent early miscarriages, fetal loss after 10 weeks’ gestation, intrauterine growth restriction, or severe preeclampsia. In rare cases, a life-threatening condition known as catastrophic APS, consisting of multiorgan thrombosis, can occur.3
Antiphospholipid Antibodies
aPLs are a heterogeneous group of antibodies directed against phospholipids and phospholipid-binding proteins. In some cases, aPLs may develop in conditions other than APS, such as in response to infections.4 aPLs may be transient or persistent (i.e., present on >2 occasions occurring >12 weeks apart).2 In some patients, aPLs frequently are slightly elevated, but at such a low level that they are not considered clinically meaningful, and they do not indicate that the patient has APS. To be clinically meaningful, aPL assessment must adhere to the guidelines for testing methods and use validated tests, results must remain persistently positive on two separate time points (>12 weeks apart), and titers must be sufficient.4
The three antibodies contributing to APS diagnosis are lupus anticoagulant (LA), anticardiolipin antibodies (aCLs), and anti–beta-2 glycoprotein I (aB2GPI) antibodies. The type of aPL, aPL titer, persistence, and presence of multiple types (double or triple vs. single) comprise the aPL profile, which is important for determining the risk of thrombotic and obstetric events and, therefore, treatment.3
Pathogenesis
It is thought that the initiating events for induction of antibodies to phospholipid-binding proteins are infections, oxidative stress, and major physical stresses (e.g., surgery, trauma). These contributors seem to induce increased apoptosis of the vessel endothelial cells and subsequent exposure of phospholipids. The binding of phospholipids to serum proteins (e.g., B2GPI, prothrombin) leads to neoantigen formation and triggers the induction of antiphospholipids. The binding of antiphospholipids to the disrupted endothelial cells results in the initiation of intravascular coagulation and thrombus formation. Other proposed mechanisms for thrombotic events and APS-related obstetric complications include complement neutrophil activation and an imbalance between type I and III interferons.5
Epidemiology
The incidence of APS is estimated to be approximately five cases per 100,000 persons per year, and its prevalence in the general population is estimated at 40 to 50 per 100,000. aPLs occur in 1% to 5% of the general population, and their prevalence increases with age.4 aCLs are present in 10% of healthy blood donors, but <1% of them are still positive 1 year after testing.2 Although one-third of patients with SLE and other autoimmune diseases possess these antibodies, only 5% to 10% of them develop APS.5
Clinical Manifestations
Common clinical features of APS include obstetric complications and venous, arterial, or microvascular thrombosis. The most common arterial events in patients with APS are stroke and transient ischemic attack. Patients with venous thromboembolism (VTE) often present with lower-extremity deep-vein thrombosis, pulmonary embolism, or both. Nonthrombotic manifestations include valvular heart disease, livedo reticularis or racemosa, nephropathy, thrombocytopenia, hemolytic anemia, and cognitive dysfunction.2 Patients who are positive for aPLs may present without symptoms. These patients are often identified during evaluation for systemic autoimmune diseases, early miscarriage, elevated activated partial thromboplastin time, or a false-positive result of a syphilis test.2
Diagnosis
The diagnosis of APS is based on the presence of >1 clinical manifestation in the setting of persistently positive aPLs. To confirm persistence, the laboratory test must be positive on two separate occasions >12 weeks apart. As discussed above, aPL test results must be interpreted cautiously, as not every patient with a positive aPL test has APS. APS is diagnosed based on careful assessment of clinically relevant health problems, clinically significant aPL profile, and additional risk factors for thrombosis.6 It should be noted that classification criteria (discussed below) are used to identify homogeneous cohorts for research purposes; diagnostic criteria are intended to identify all patients with a given disorder regardless of unusual clinical presentations.2
Classification
In 2023, the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) jointly published updated classification criteria for APS. APS classification was previously based on the Sapporo criteria, which were published in 1999 and revised in 2006. Since the 2006 revision, advances have led to a better understanding of APS, including improved characterization of aPL-associated nonthrombotic clinical manifestations, identification of the role played by traditional thrombosis risk factors in aPL-positive patients, and risk stratification by aPL profile. Additionally, the revised Sapporo criteria were criticized for not incorporating evidence-based definitions. The 2023 ACR/EULAR classification includes definitions of clinical and laboratory criteria and definitions of high-risk profiles for VTE and cardiovascular disease, as well as a scoring system (FIGURE 1). When the 2023 classification was compared with the 2006 Sapporo classification, the new criteria had a specificity of 99% versus 86% and a sensitivity of 84% versus 99%. The 2023 ACR/EULAR criteria can help ensure future higher-quality, risk-stratified epidemiologic studies and clinical trials on APS, leading to improved patient care and management recommendations.7

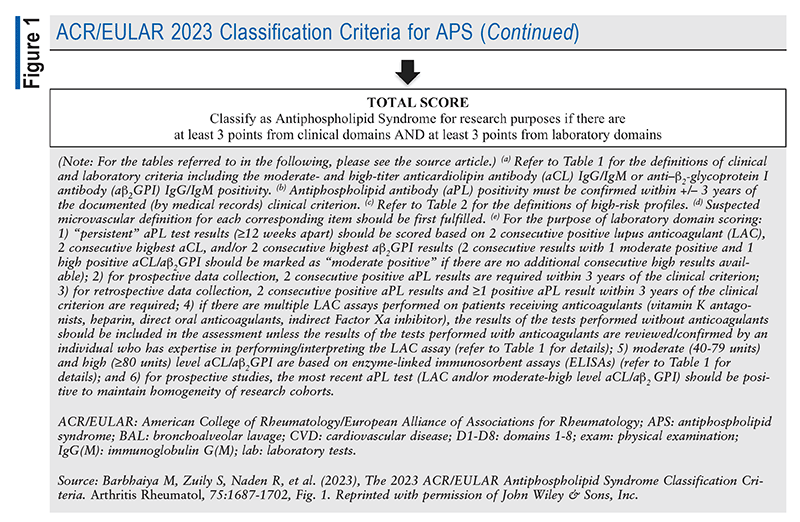
Patients are classified as having APS if they fulfill the criteria listed in FIGURE 1. Patients must fulfill the entry criteria (having >1 clinical criterion and 1 laboratory criterion within 3 years of each other) and must accumulate >3 points from clinical domains and 3 points from laboratory domains. The six clinical domains include macrovascular VTE, macrovascular arterial thrombosis, microvascular manifestations, pregnancy morbidity, cardiac-valve abnormalities, and hematologic abnormalities. Clinical criteria do not count if there is an equally likely or more likely explanation than APS. Laboratory criteria include the result of the LA test and the presence and quantity of aCL and aB2GPI in the blood. Two positive tests >12 weeks apart are necessary for measurement of aCL, and persistence factors into the weighting of LA.7
Management
Owing to heterogeneity among studies regarding the laboratory and clinical criteria used to define APS and its treatment approaches, it is often difficult to know the best treatment approach.2 It is important to identify and address factors that increase thrombosis risk, such as high-risk aPL profile, coexistence of other systemic autoimmune diseases, and traditional risk factors for cardiovascular disease (e.g., smoking, hypertension, diabetes, hyperlipidemia).2,3 Vitamin K antagonists (VKAs) continue to be the standard of care for long-term anticoagulation in most APS patients despite the introduction of direct oral anticoagulants (DOACs) to the market.2
Primary Thrombosis Prevention
The use of low-dose aspirin (LDA) for primary thrombosis prevention in APS is controversial based on the low quality of evidence and the lack of prospective data showing that LDA is effective.2 A report from the 16th International Congress on Antiphospholipid Antibodies recommends consideration of LDA for primary prevention of thrombosis on a case-by-case basis in certain patient groups. LDA should be considered in asymptomatic aPL carriers with or without SLE and patients with prior obstetric APS who have persistent LA, double or triple aPL positivity, or persistently high aPL titer. LDA may be considered in asymptomatic aPL carriers with or without SLE or in patients with prior obstetric APS with any other aPL phenotypes. Patients and providers should discuss the risks and benefits, including patient-related risk factors for arterial thrombosis, VTE, bleeding, and upper gastrointestinal reflux disease.8 According to EULAR guidelines, prophylaxis with LDA at a dosage of 75 mg to 100 mg daily is recommended in patients with a high-risk profile and may be considered in patients with a low-risk profile.5
Further research is needed in the form of randomized, controlled trials studying the potential role of LDA or other antiplatelet agents for primary prevention of thrombosis in asymptomatic aPL-positive patients.8
Secondary VTE Prevention
In patients with APS defined by venous thrombosis, it is recommended to initially treat with unfractionated heparin or low-molecular-weight heparin (LMWH) followed by long-term anticoagulation with a VKA such as warfarin. The recommended international normalized ratio (INR) goal is 2 to 3; an INR goal of 3 to 4 was associated with fewer thrombotic events in two retrospective studies; however, based on the results of two randomized, controlled trials, this goal was not shown to reduce the risk of recurrent thrombosis.2 EULAR guidelines recommend an INR goal of 2 to 3 for patients with APS and unprovoked venous thrombosis.3
Long-term anticoagulation is recommended in most patients who have persistent aPLs and who had an otherwise unprovoked VTE. However, the benefit of prolonged anticoagulation is less clear in patients who are positive for aPLs but had a provoked thrombus and in patients whose laboratory tests for aPLs become negative over time.2
Secondary Arterial Thromboembolism Prevention
There is less consensus about the optimal management of APS patients who have experienced an arterial event.2 Patients with moderate-risk to high-risk aPL profiles are often treated with warfarin (INR goal, 2-3). Some clinicians prefer higher-intensity warfarin therapy (INR goal, 3-4) for patients with arterial thrombosis, based on the paucity of data on patients with arterial thrombosis in randomized, controlled trials comparing different intensities of warfarin therapy.2
Because of a lack of consensus, guidelines from the 13th International Congress on Antiphospholipid Antibodies provided a nongraded recommendation, stating that patients with APS and arterial thrombosis should be treated with warfarin at an INR >3 or with combined antiaggregant-anticoagulant therapy (INR goal, 2-3). However, some members of the task force were of the opinion that antiaggregant therapy or anticoagulant therapy alone (INR goal, 2-3) would be equally valid in this setting.6
The 16th International Congress on Antiphospholipid Antibodies report found insufficient evidence for strong recommendations concerning the use of LDA for secondary prevention following a first APS-related arterial thrombosis; however, it stated that LDA use could be considered in combination with standard-intensity VKA therapy (INR goal, 2-3).8 EULAR guidelines recommend VKA therapy after the first arterial thrombosis in APS patients, with a recommended INR goal of 2 to 3 or 3 to 4 based on the patient’s risk for bleeding and recurrent thrombosis; the guidelines add that treatment with a VKA (INR goal, 2-3) plus LDA may be considered.3
Further research is needed in the form of randomized, controlled trials to define the role of LDA or other antiplatelet agents, in combination with anticoagulation, in thrombotic APS patients.8
Treating Recurrent Thromboemboli
There is limited high-quality evidence supporting any management strategy when the use of warfarin fails despite the presence of a therapeutic INR. Options include higher-intensity warfarin therapy (target INR, 3-4); the addition of LDA, hydroxychloroquine (HCQ), or a statin; the use of a different anticoagulant; or some combination of these approaches.2
According to the 16th International Congress on Antiphospholipid Antibodies report, LDA may be considered in combination with anticoagulation in patients who develop recurrent arterial or venous thrombosis on a standard-intensity VKA. The report also states that the addition of HCQ and statins may be considered as an adjunct to antithrombotic therapy in anticoagulant-refractory thrombotic APS.8
EULAR guidelines state that investigation of and education about medication adherence and frequent INR testing should be considered in APS patients with recurrent thrombosis despite treatment with a VKA (INR goal 2-3). Additionally, if the target INR of 2 to 3 was achieved, potential strategies after evaluation for other potential causes include adding LDA, increasing the INR goal to 3 to 4, or switching to LMWH.3
Direct Oral Anticoagulants
DOACs have advantages over warfarin, including fewer drug interactions and the fact that they are prescribed in a fixed dose and tend to have a predictable anticoagulant effect without the need for routine anticoagulation monitoring.7 Although most of the commercially available DOACs compare favorably with warfarin for treatment of VTE and prevention of stroke in patients with atrial fibrillation, there is a lack of published data on the use of DOACs for highly prothrombotic states such as APS, and available data indicate that the DOACs should be avoided in most APS patients, based on the results of several studies.2,8
The 16th International Congress on Antiphospholipid Antibodies report recommends first-line therapy with a VKA in most patients. DOACs should be avoided in patients with arterial thrombosis and in thrombotic APS patients with small-vessel thrombosis or aPL-related cardiovascular disease. Following a first episode of VTE, continuation of a DOAC may be considered in patients found to have single- or double-positive aPLs while awaiting confirmation of persistence of aPLs (based on testing after >12 weeks). Shared decision-making should be employed; the provider should inform the patient about the uncertainties of anticoagulant selection, and they should discuss the risks and benefits of DOAC use. If a DOAC is considered, aB2GPI testing should be performed to distinguish the presence of double-positive versus triple-positive aPLs. Patients with triple-positive aPLs who were initially started on a DOAC should be switched to warfarin or an alternative VKA. If the patient declines, the DOAC may be continued, with clinical surveillance, including brain MRI, to identify ischemic lesions. DOACs should not be used in APS patients with recurrent thrombosis while on a standard-intensity VKA.8
For patients with venous thrombosis, EULAR guidelines recommend against using rivaroxaban in patients with triple aPL positivity due to the high risk of recurrent events; however, they note that DOACs may be considered in certain patients, such as those with contraindications to VKAs or those unable to achieve target INR while on a VKA despite adherence. EULAR guidelines also recommend against rivaroxaban use in arterial-thrombosis patients with triple aPL positivity. The use of DOACs in patients with definite APS and arterial events is not recommended, based on the high risk of recurrent thrombosis.3
Future research is needed on the potential role of DOACs in APS. Studies should take into account APS’s heterogeneity and the fact that thrombotic risk is influenced by the clinical and laboratory APS phenotype. A registry of patients with APS on DOAC therapy is currently being established by the International Society on Thrombosis and Haemostasis to ensure consistency of data collection and the provision of safety information to APS patients.8
Adjunctive Therapies
Most patients with thrombotic APS respond to anticoagulation, but some patients continue to experience clinical events despite anticoagulation. There may be a role for agents such as HCQ, statins, and vitamin D as alternative therapies for APS (discussed below). Other potential therapies include biologics (rituximab, belimumab, and anti–tumor necrosis factor drugs), complement activation, treatments based on peptides of B2GPI, coenzyme Q10, adenosine receptor agonists, and agents with adenosine-potentiating properties.8
Hydroxychloroquine
Antithrombotic effects of HCQ, which is a standard treatment for SLE, include reversal of aPL-induced platelet activation and potentially beneficial effects on cholesterol. A retrospective study showed that HCQ may reduce aPL titers in primary-APS patients, and a prospective study found that HCQ use was associated with a reduction in aPL titers. The 16th International Congress on Antiphospholipid Antibodies report notes that the addition of HCQ may be considered adjunctively to antithrombotic treatment in patients with anticoagulant-refractory APS. HCQ may also be considered in patients with obstetric APS that is refractory to standard treatment. EULAR guidelines state that HCQ may be used in women with refractory obstetric APS.3
Further research is needed to ascertain the potential benefit of HCQ in primary APS and in patients without SLE who have aPLs or thrombotic APS.7 EULAR guidelines call for more research on the role of HCQ in primary thrombosis prevention.3
Statins
The 16th International Congress on Antiphospholipid Antibodies report states that statins may be beneficial in the primary and secondary prevention of arterial thrombosis in patients with aPLs/APS but cannot be recommended in patients in the absence of hyperlipidemia. The report adds that statins may be considered adjunctively to antithrombotic treatment in anticoagulant-refractory thrombotic APS patients.8
Further research is needed to define the potential utility of statins for primary prevention and in patients with thrombotic APS.3,8
Vitamin D
Low vitamin D levels are associated with thrombotic manifestations (venous and arterial) in APS patients. It was found that vitamin D insufficiency (serum level <30 ng/mL) occurs in up to 70% of patients with APS and vitamin D deficiency (serum level <10 ng/mL) occurs in 11% to 50%. In studies, vitamin D levels were lower in APS patients than in controls, and levels in APS patients with thrombotic manifestations were significantly lower than in APS patients with only obstetric manifestations.8 The 16th International Congress on Antiphospholipid Antibodies report recommends correcting vitamin D deficiency and insufficiency in all patients based on guidelines for the general population.8
Further research is needed on the role of vitamin D deficiency in aPL-positive patients and to clarify the therapeutic value of supplementation, including treatment goals and recommended dosing. More studies are required to determine whether vitamin D deficiency in APS patients is caused by disease pathogenesis or disease activity or is an incidental modifying factor.8
Conclusion
APS is an autoimmune disorder characterized by thrombosis or pregnancy morbidity in conjunction with the presence of persistent aPLs (LA, aCLs, and aB2GPIs). VKAs remain the standard of care for APS management in most cases; DOAC use is not generally recommended, owing to limited evidence. Aspirin use is controversial and depends on individual risk. More research is needed to establish therapeutic recommendations for APS treatment.
REFERENCES
1. Lim W. Antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2013;2013:675-680.
2. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010-2021.
3. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296-1304.
4. Hospital for Special Surgery. Classification versus diagnosis of antiphospholipid syndrome: how are they different? www.hss.edu/conditions_top-ten-points-classification-versus-diagnosis-antiphospholipid-syndrome-how-are-they-different.asp. Accessed January 10, 2024.
5. Moutsopoulos HM, Mavragani CP. Antiphospholipid syndrome. In: Loscalzo J, Fauci A, Kasper D, et al, eds. Harrison’s Principles of Internal Medicine. 21st ed. New York, NY: McGraw Hill; 2022.
6. Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on Antiphospholipid Antibodies. Lupus. 2011;20(2):206-218.
7. Barbhaiya M, Zuily S, Naden R, et al. The 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol. 2023;75(10):1687-1702.
8. Cohen H, Cuadrado MJ, Erkan D, et al. 16th International Congress on Antiphospholipid Antibodies Task Force report on antiphospholipid syndrome treatment trends. Lupus. 2020;29(12):1571-1593.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






