US Pharm. 2023;48(8):HS-11-HS-16.
ABSTRACT: Adverse drug reactions (ADRs) in hospitalized pediatric patients are a significant concern, with potential short- and long-term consequences. ADRs can range from mild to severe, and children are particularly vulnerable due to developing organ systems and difficulties in effectively communicating symptoms. Polypharmacy, off-label drug use, chronic conditions, and prolonged hospital stays increase the risk of ADRs. Collaborative efforts among healthcare providers and medication therapy management play crucial roles in preventing and managing ADRs. Pharmacists should ensure that prescribed dosages are accurate for the child’s age, weight, and medical condition. Involving patients and families in medication education further enhances medication safety for pediatric patients.
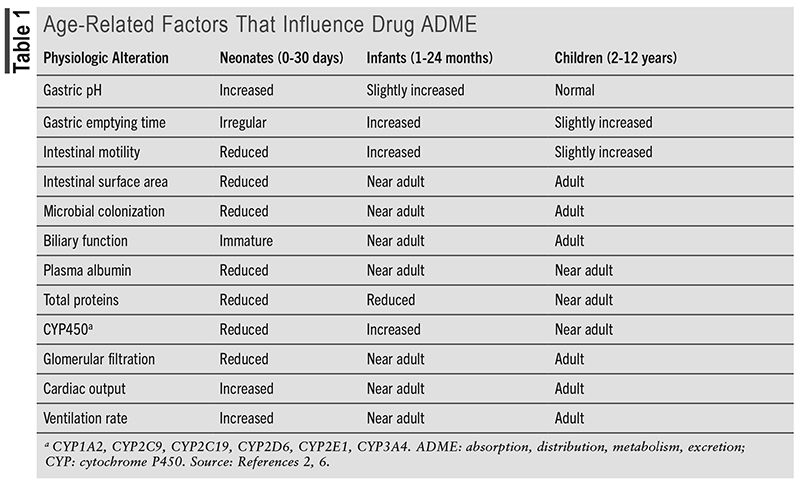
An adverse drug reaction (ADR) is an unintended, harmful reaction to a medication that is prescribed appropriately and a subset of adverse drug events (ADEs) that are an unintended harmful reaction to a medication that may or may not be prescribed appropriately.1,2 Although some ADRs may be idiosyncratic, which are unpredictable and often more severe, most are an extension of the pharmacology of the drug.3 ADRs can manifest across a spectrum of severity, ranging from mild symptoms, such as dyspepsia, to severe reactions like anaphylaxis or fatality. Any patient may experience an ADR, but children, especially the young, are more vulnerable due to developing organ systems.3 TABLE 1 describes age-dependent changes that influence drug absorption, distribution, metabolism, and excretion. For hospitalized pediatric patients, experiencing an ADR may prolong hospital stays, increase healthcare costs, and even result in death.3-6 ADRs are a common occurrence in the pediatric population, and unlike adult medications with standard doses, children often require weight-based dosing, which may lead to calculation errors. Children may also lack the ability to communicate symptoms effectively, making diagnosis of an ADR challenging. This article provides an overview of ADRs in hospitalized pediatric patients. Additionally, this article will explore strategies for preventing and managing ADRs, the role of pharmacists in minimizing ADRs, and the importance of patient and family involvement in preventing ADRs.
Consequences of ADRs have short- and long-term effects. Long-term patients may have cognitive and developmental delays, organ damage, and chronic health issues, all of which can be a burden on the family and lead to direct and indirect medical costs.5 The first step in the prevention of ADRs is knowing the risk factors involved. As pharmacists, it is important to understand the individual risk factors for ADRs and management strategies to minimize harm and improve patient outcomes.
Prevalence and Common Medications
Capturing the true incidence of ADRs in children is difficult, as many go unreported. In addition, the lack of standardization for reporting ADRs hinders data collection and assessment.2 Nevertheless, results from various observational and systematic reviews show an incidence of ADRs in hospitalized children of 0.4% to 17.7%.4,7-10 This incidence also varies according to the patient’s comorbidities. Patients with liver or renal dysfunction, who are immunocompromised, or who have chronic diseases or a critical illness are at higher risk of ADRs. One prospective observational cohort study of 5,118 children (6,601 admissions) who spent at least 48 hours as an inpatient showed an ADR incidence (of definite or probable ADR) per patient of 17.7% (95% CI, 16.7% to 18.8%).11 When stratifying results by number of admissions, children admitted more than once had a higher incidence of ADRs compared with those admitted only once, 18.0% (95% CI, 16.4% to 19.6%) versus 14.7% (95% CI, 13.7% to 15.9%), respectively.11 Interestingly, over 50% of the ADRs were linked to opioids or medications used during general anesthesia.11 Risk factors for experiencing an ADR were age on admission (younger patients are at greater risk), number of medications, receiving general anesthesia, and being an oncology patient.11
A retrospective analysis assessed 3,690 ADRs and found that children were over four times as likely as adults (excluding geriatrics) to experience an ADR, 9.5% versus 2.1%.3 The most common medications implicated were antimicrobials, blood products, and those affecting the CNS.3 Another retrospective study of pediatric inpatient hospital records from 16 teaching and nonteaching hospitals found ADRs reported in 10.9% (414/3,790) of records, in which over half were considered preventable.12 The most common ADRs were associated with hospital-acquired infections (18.6%), IV line complications (14.5%), gastrointestinal related (12.8%), and respiratory related (11.8%).12
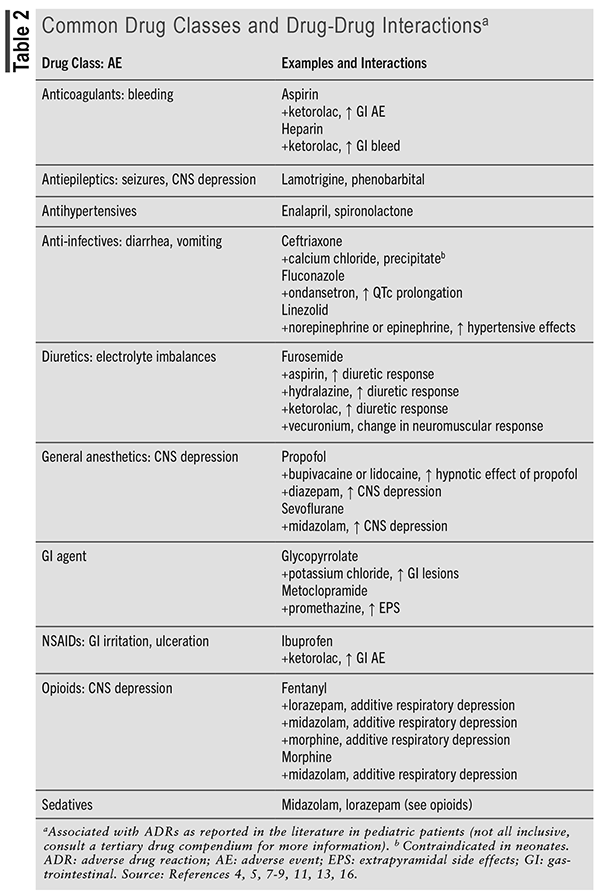
Common medications involved in ADRs include analgesics, anti-infective agents, antineoplastics, antipsychotics, dopamine antagonists, corticosteroids, immunomodulators, neurologic agents, gastrointestinal agents, and cardiovascular agents.5,9,13 TABLE 2 describes common drug classes and drug-drug interactions identified in pediatric hospitalized patients that increase the risk of ADRs.
Risk Factors
Despite advancements in technology and increased safety measures within hospitals, pediatric inpatient ADRs remain high. Several factors contribute to a child’s increased risk of experiencing ADRs. Common risk factors observed during inpatient stays include polypharmacy, prolonged length of stay, presence of chronic conditions or comorbidities, and the use of off-label medications.7,8,10
Polypharmacy
Polypharmacy can heighten the risk for ADRs, especially considering that a significant portion of medications prescribed to children are used off-label. As with adults, children are placed at risk for drug-drug interactions through their exposure to multiple medications.5,8,13,14 More than 50% of pediatric patients treated as inpatients are exposed to greater than five medications during their stay, which increases the likelihood of drug-drug interactions.15 In addition, patients with chronic conditions or those with longer lengths of stay may be prone to polypharmacy.14 A retrospective cohort study that evaluated 498,956 pediatric hospital stays found a 2.5-fold increase in ADRs in pediatric patients with more than three chronic conditions.13 In addition, the incidence of ADRs was higher in children with chronic conditions (>3 body systems affected) versus those without any chronic condition, 33.9% versus 14.0% per 1,000 patient days.12
Length of Stay and Chronic Illnesses
Patients hospitalized for chronic conditions often have longer treatment durations and, thus, an increased risk of experiencing an ADR. Results from a retrospective study that evaluated potential drug-drug interactions in children admitted to 43 children’s hospitals showed that of the 498,959 unique hospitalizations, 49% were exposed to ≥1 potential drug-drug interaction, of which 5% were contraindicated, 41% considered major, and 28% considered minor according to Micromedex.13 Similar results were shown in pediatric patients admitted to intensive care units. A retrospective cohort showed that pediatric patients hospitalized in the intensive care unit of 51 free-standing pediatric hospitals were exposed to 10 distinct drugs each hospital day and to 20 during their stay, which increased the potential for drug-drug interactions and ADRs.16 Results showed that 75% (41,034/54,549) were exposed to ≥1 potential drug-drug interaction, of which 0.8% were contraindicated, 51.1% major, and 42.9% moderate severity of interaction, according to Micromedex (TABLE 2).16
Off-Label Drug Use
Healthcare practitioners often rely on clinical trial data that may not adequately cover the patient’s age or other relevant factors. Consequently, medications prescribed for children based on adult data may increase the risk of ADRs. It is estimated that 50% of drugs on the market lack specific pediatric labeling.8 Results of a prospective observational study of 320 pediatric inpatients showed that 70% (1,152/1,645) of medications were prescribed off-label, with most (55.6%) due to a variation in dose, and that 10.9% of patients reported an ADR.17 Off-label drug use is a challenging dilemma facing healthcare professionals to determine what is deemed safe and appropriate in the pediatric population.
Prevention of ADRs in Pediatric Populations
As medication therapy becomes increasingly complex, the role of pharmacists in preventing ADRs in pediatric patients is more critical than ever. Through collaboration, medication therapy management, and education, pharmacists can help reduce the risk of ADRs and improve medication safety in children.
Collaboration among healthcare providers is particularly important in pediatric healthcare, where multiple providers may be involved in a child’s care. Effective communication and collaboration among healthcare teams can improve medication safety by minimizing the potential for medication errors and ADRs. It also ensures that all healthcare providers are informed about the child’s medication history, current medications, and potential medication interactions. The US Pharmacopeia (USP) Medication Errors Reporting Program revealed higher rates of medication errors in pediatric patients than in adults (31% vs. 13%).18 While not all medication errors will lead to ADRs, it is an important role for pharmacists to stop preventable drug errors. Preventive strategies in prescribing and administration of medications can help reduce the incidence of ADRs in pediatric patients. These include using pediatric-specific dosing guidelines, conducting medication reconciliation, and utilizing medication order sets.
In conjunction with the American Society of Health-System Pharmacists, the Pediatric Pharmacy Advocacy Group recommends the following to meet the needs of pediatric patients19:
• Establish specialized pediatric pharmacy expertise within hospitals and health systems to optimize medication therapy for children.
• Develop and implement pediatric-specific medication dosing, compounding, and formulation guidelines.
• Integrate pediatric pharmacists as essential members of healthcare teams to ensure safe and effective medication use in pediatric patients.
• Foster collaboration among healthcare professionals to create and enforce pediatric-specific medication policies and protocols.
• Address challenges related to medication administration and adherence in pediatric patients through proactive measures.
• Promote medication safety by actively monitoring for ADRs in pediatric patients and providing education to healthcare providers, patients, and families.
• Support ongoing professional development and training opportunities for pediatric pharmacists to stay up to date with advancements in pediatric pharmacotherapy.
• Allocate necessary infrastructure and resources to facilitate pediatric pharmacy services within hospitals and health systems.
• Encourage pediatric pharmacists to engage in research, quality-improvement initiatives, and advocacy efforts to advance pediatric pharmacy practice.
Medication therapy management (MTM) can be particularly useful in identifying potential medication-related problems and preventing ADRs. Pharmacist-led MTM can improve medication safety in pediatric populations by identifying ADRs, managing potential medication interactions, and optimizing medication regimens. Although many hospitalized patients require a multitude of medications, polypharmacy is preventable, and reducing excessive prescribing may reduce the potential for an ADR.
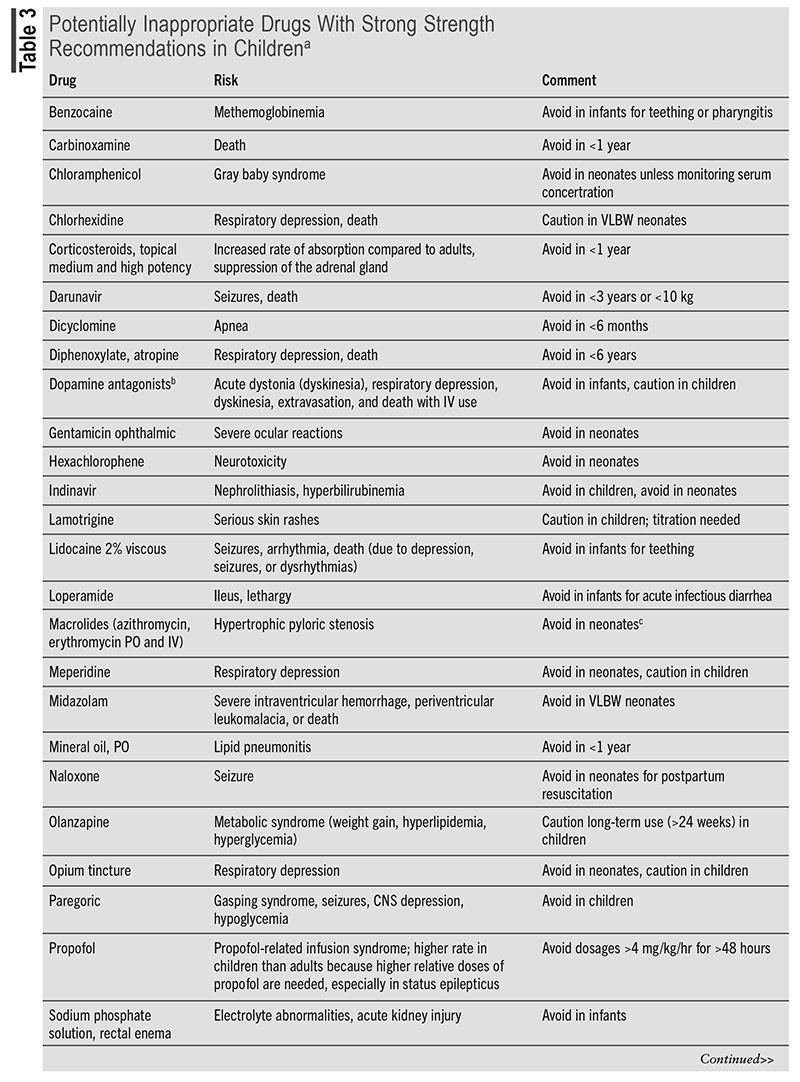
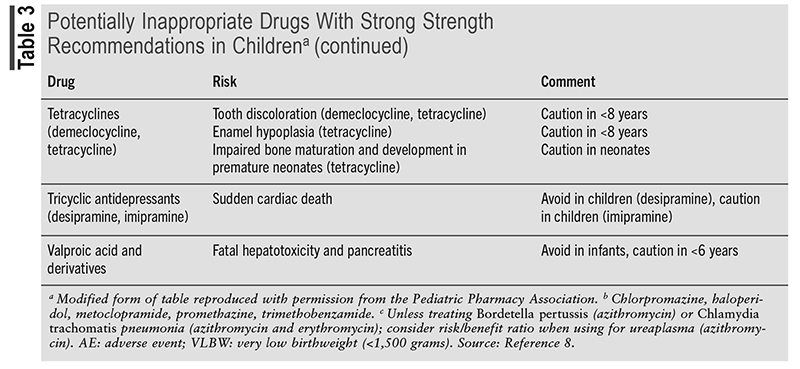
Pharmacovigilance and continuous monitoring of patients are essential in preventing and managing ADRs. A clinical approach to ADRs is a stepwise system called the Five As. The Five As consist of Appreciation (understanding of the potential for ADRs), Assessment (data collection), Analysis (of the data collected), Assistance (supportive and symptomatic care), and Aftermath (communication with patient/family member).20 Following the Five As encourages evaluation of all aspects of the reaction, the appropriate management, and follow-up. Members of the Pediatric Pharmacy Association created the Key Potentially Inappropriate Drugs in Pediatrics (KIDs) list that includes medications that should be not used or used with caution in pediatric patients.8 The KIDs list provides a compilation of drugs based on the severity of each ADR commonly experienced. TABLE 3 presents a condensed list of KIDs drugs. Through pharmacovigilance and medication management, it is crucial for pharmacists to identify medications that may put a child at risk for ADRs.
Patient and family involvement is essential in preventing ADRs in pediatric patients. This includes educating patients and families about medications and encouraging them to ask questions and raise concerns. Additionally, empowering patients and families to be advocates for their own healthcare can also help prevent ADRs, especially with discharge medications.7
Conclusion
ADRs are a significant concern in hospitalized pediatric patients. Healthcare providers must remain vigilant and proactive to minimize harm and improve patient outcomes. Understanding ADRs and management strategies is key to achieving this goal. Pharmacists play a crucial role in preventing ADRs by collaborating with healthcare providers, managing medication therapy, and providing education, thereby improving medication safety. Effective communication and collaboration among healthcare teams, along with preventive strategies such as using pediatric-specific dosing guidelines and medication reconciliation, can help reduce the incidence of ADRs in children.
REFERENCES
1. Zed PJ, Haughn C, Black KJL, et al. Medication-related emergency department visits and hospital admissions in pediatric patients: a qualitative systematic review. J Pediatr. 2013;163(2):477-483.
2. Sandritter TL, Jones BL, Kearns GL, Lowry JA. Principles of drug therapy. In: Kliegman RM, St. Geme JW III. Nelson Textbook of Pediatrics. Elsevier Inc.; 2020:445-456.
3. Amin S, Shah S, Desai M, et al. An analysis of adverse drug reactions in extremes of age group at tertiary care teaching hospital. Perspect Clin Res. 2018;9(2):70-75.
4. Tripathy R, Das S, Das P, et al. Adverse drug reactions in the pediatric population: findings from the adverse drug reaction monitoring center of a teaching hospital in Odisha (2015-2020). Cureus. 2021;13(11):e19424.
5. Sugioka M, Tachi T, Mizui T, et al. Effects of the number of drugs used on the prevalence of adverse drug reactions in children. Sci Rep. 2020;10(1):21341.
6. Tanem JM, Scott JP. Common presentations of rare drug reactions and atypical presentations of common drug reactions in the intensive care unit. Crit Care Clin. 2022;38(2):287-299.
7. Gupta S, Zaki SA, Masavkar S, Shanbag P. Causality, severity, and avoidability of adverse drug reactions in hospitalized children: a prospective cohort study. Cureus. 2023;15(1):e33369.
8. Meyers RS, Thackray J, Matson KL, et al. Key potentially inappropriate drugs in pediatrics: the KIDs list. J Pediatr Pharmacol Ther. 2020;25(3):175-191.
9. Andrade PHS, Lobo IMF, da Silva WB. Risk factors for adverse drug reactions in pediatric inpatients: a cohort study. PLoS One. 2017;12(8):e0182327./
10. Wimmer S, Neubert A, Rascher W. The safety of drug therapy in children. Dtsch Arztebl Int. 2015;112(46):781-787.
11. Thiesen S, Conroy EJ, Bellis JR, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children—a prospective observational cohort study of 6,601 admissions. BMC Med. 2013;11:237.
12. Stockwell DC, Landrigan CP, Toomey SL, et al. Adverse events in hospitalized pediatric patients. Pediatrics. 2018;142(2):e20173360.
13. Feinstein J, Dai D, Zhong W, et al. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):e99-e108.
14. Bogler O, Roth D, Feinstein J, et al. Choosing medications wisely: is it time to address paediatric polypharmacy? Paediatr Child Health. 2019;24(5):303-305.
15. Antoon JW, Hall M, Herndon A, et al. Prevalence of clinically significant drug-drug interactions across US children’s hospitals. Pediatrics. 2020;146(5):e20200858.
16. Dai D, Feinstein JA, Morrison W, et al. Epidemiology of polypharmacy and potential drug-drug interactions among pediatric patients in intensive care units of U.S. children’s hospitals. Pediatr Crit Care Med. 2016;17(5):e218-e228.
17. Saiyed MM, Lalwani T, Rana D. Is off-label use a risk factor for adverse drug reactions in pediatric patients? A prospective study in an Indian tertiary care hospital. Int J Risk Saf Med. 2015;27(1):45-53.
18. D’Errico S, Zanon M, Radaelli D, et al. Medication errors in pediatrics: proposals to improve the quality and safety of care through clinical risk management. Front Med (Lausanne). 2022;8:814100.
19. Eiland LS, Benner K, Gumpper KF, et al. ASHP–PPAG guidelines for providing pediatric pharmacy services in hospitals and health systems. J Pediatr Pharmacol Ther. 2018;23(3):177-191.
20. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






