US Pharm. 2023;48(8):17-32.
ABSTRACT: The precipitous growth of pediatric obesity worldwide is having deleterious effects. A change in the landscape of daily activities, mainly nutrition and physical activity, has the strongest correlation to an unhealthy BMI. Early education on healthy living for youth and caretakers can aid in the prevention and treatment of pediatric obesity. At present, pharmacologic agents should be reserved for youth aged 12 years or older with a diagnosis of obesity and tailored based on comorbid conditions. Given the small amount of evidence and limited follow-up periods, more research is needed to guide selection and duration of medication use.
The February 2023 release of much-needed practice guidelines to address pediatric obesity should serve as a wake-up call to the healthcare community and patients alike.1 Moving like a silent pandemic across all nations, the prevalence of pediatric overweight and obesity worldwide has risen from 4% in 1975 to just over 18% in 2016 (for individuals aged 5-19 years).2 In the United States, reports from the National Center for Health Statistics showed that about 14.4 million children and adolescents aged 2 to 19 years were obese, disproportionally affecting racial/ethnic minorities and the socioeconomically challenged.3 Globally, more deaths are linked to issues arising from overweight and obesity than underweight.
While all estimates show a rise in overweight and obesity within the pediatric population, the definition of who meets those criteria differs based on the reference (TABLE 1).2,4,5 Concerns have been raised in the literature regarding the specificity and sensitivity of BMI cutoffs in determining overweight and obesity in the pediatric population that are similar to BMI criteria for adults.6 Additional methods of assessment in the clinical environment include waist circumference and skin-fold thickness, waist circumference being a more accurate assessment of central obesity. Short- and long-term consequences of pediatric overweight and obesity include hypertension, dyslipidemia, insulin resistance, type 2 diabetes, and nonalcoholic fatty liver disease.1 These patients are also at higher risk for poor psychological and emotional health, presenting with increased stress, depressive symptoms, and low self-esteem.
CAUSES AND RISK FACTORS
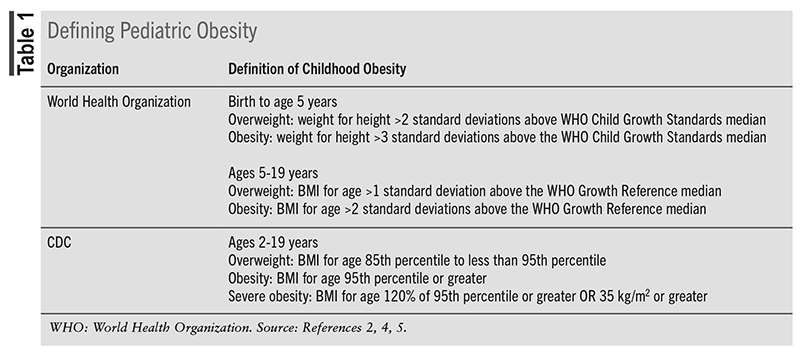
The increased incidence of pediatric overweight and obesity is a result of patient-specific and environmental variables.1,6 The role of genetics and endocrine and basal metabolic rate in the development of pediatric obesity is still under investigation, but they likely do not account for the sharp increase of children who meet the diagnosis. Changes in social norms, food consumption, and energy expenditure have been deemed the primary causes of the current pandemic (FIGURE 1). The landscape around food has moved away from healthy eating, with an increase in accessibility and availability of low-nutrient foods. Dining out, fast-food restaurants, high costs of fresh food, larger than necessary food portions, and sweet beverages are just a few of the negative contributors. High-calorie diets are also being met with limited energy expenditure due to an increase in sedentary behavior; calories not used then become adipose tissue.
COMPREHENSIVE EVALUATION
Annual visits to a pediatrician or family medicine physician should be completed for all children.1 Healthcare providers should be sensitive to avoid propagating social stigma, as this can negatively affect children participating in healthcare. For a child diagnosed as overweight or obese, a complete history, review of systems, and physical examination should be conducted. Important considerations to note would be indicators of a genetic or health cause, dietary and exercise patterns, and medication history. Examples of medications that are associated with weight gain include atypical antipsychotics, antidepressants, steroids, anticonvulsants, antihypertensives, birth control agents including injected forms, and medications used in diabetes mellitus. Overweight and obesity have been shown to correlate with the development or coexistence of a multitude of chronic diseases, such as hypertension, dyslipidemia, prediabetes and diabetes, and nonalcoholic fatty liver disease (NAFLD). With the exception of blood pressure, screening for these conditions should be conducted in children aged older than 10 years.
TREATMENT
Motivational Interviewing
Motivational interviewing (MI) is a form of counseling that identifies a patient’s motivation for change and aids in the resolution of ambivalence.1 In the context of obesity, it can be utilized to guide families in identifying a behavior to change based on what the parent(s) or child feels is important and which can be accomplished. The process of motivational interviewing was first coined in the early 1980s by William R. Miller, PhD, and describes a four-step process that includes engaging, focusing, evoking, and planning.
To begin the process of MI, the pediatrician must establish a strong relationship with the patient and their family. If there is any disconnect in the patient-provider relationship, obesity treatment may be more likely to fail, as engaging is the basis of MI. Therefore, pediatricians and primary healthcare providers (PHCPs) must be readily accessible to patients and be conscious of other challenges the family may present with, such as financial constraints and other health concerns.1 Given the longitudinal nature of this process, the focusing stage of MI becomes more relevant as the patient ages. As patients mature, they will begin to identify behaviors to change more independently; however, collaboration must continue to occur between the provider, patient, and patient’s family.
The evocation phase further advances patients’ autonomy, as they are now adolescents capable of evaluating their own goals and assessing their readiness to change. Finally, in the planning phase, patients should be made aware of all resources and support available to them. During this phase, pediatricians and PHCPs must be diligent in following their patients’ progress and offering guidance throughout as setbacks and relapses may occur more frequently.
Intensive Health Behavior and Lifestyle Treatment
Intensive health behavior and lifestyle treatment (IHBLT) is now a vital component in the treatment of pediatric obesity for children aged 6 years and older and those aged 2 to 5 years with overweight or obesity. IHBLT programs provide and support families with educational activities focused on nutrition and physical activities for 3 to 12 months.1 These programs are designed to promote healthy changes that improve weight status, comorbidities, and long-term health. At minimum, families should receive 26 hours of face-to-face multicomponent treatment for clinically significant outcomes to be observed at the end of the 3- to 12-month course. This 26-hour minimum is the driving factor, as the intensity (or dose) of IHBLT is measured in face-to-face patient contact hours, and the number of hours is directly proportional to the reduction in BMI.
Although not universally available, there are some IHBLT programs readily available for use by pediatricians and PHCP offices. However, when a program is not available, pediatricians and PHCP offices should work in conjunction with registered dietitians, other medical or healthcare systems, and community organizations to provide and/or establish an intensive program. In particular, clinic-community partnerships have shown easy implementation and good engagement in low-income and racially diverse populations. Regardless of origin, all IHBLT programs should offer patients at least 26 hours of face-to-face, family-based multicomponent lessons for a course of 3 to 12 months. In all effective studies, having the family unit or at least one parent involved in treatment was associated with higher success rates with a sustained reduction in BMI than those interventions without family involvement. Overall, these programs must be multifactorial to cover all aspects of tackling obesity.
Pharmacotherapy
The 2023 pediatric obesity guidelines recommend the initiation of weight-loss pharmacotherapy in conjunction with IHBLT in children aged 8 to 11 years who require more intense management of their obesity.1 Treatment recommendations should be based on risk factors, medication indication and side effects, and benefits, giving special consideration to children who are classified as severely obese, those with comorbid conditions, and children of older age. Although the use of pharmacotherapy as a weight-loss tool is an emerging field, there is still insufficient evidence for medication use in children aged younger than 12 years in whom obesity is the only indication. Pharmacotherapy for pediatric obesity is shown in TABLE 2.7
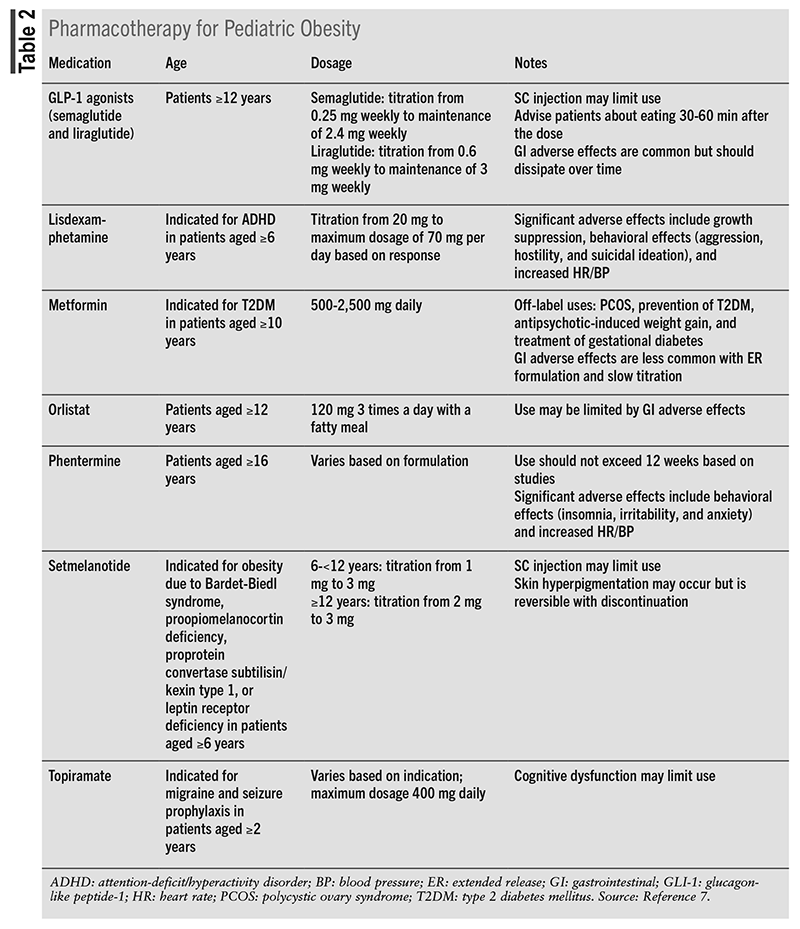
One of the most notable antidiabetic agents, metformin, which is classified as a biguanide, is approved for use in type 2 diabetes patients aged 10 years and older and has several off-label uses, including polycystic ovary syndrome, prevention of type 2 diabetes, antipsychotic-induced weight gain, and treatment of gestational diabetes.7 Two dosing formulations for metformin exist: immediate- and extended-release, starting at 500 mg with a total daily dosage of 2,500 mg. Although its use is not currently FDA approved for weight loss, multiple studies of metformin’s efficacy as a weight-loss agent exist, but they are not without conflicting data. The most efficacious studies resulted when metformin was used in higher doses in conjunction with marked lifestyle and behavioral therapy in children classified as severely obese and when other indications for the use of metformin were present. However, with the increased dosing of metformin, more adverse effects were present, including flatulence, diarrhea, nausea, and bloating. Notably, taking metformin with a meal can reduce these adverse effects. Due to the current gap in efficacy data, metformin may be considered as an add-on agent to IHBLT when other pre-existing indications are present.1
Currently approved for management of obesity in children aged 12 years and older, orlistat is available as an OTC or prescription product and acts through inhibition of gastric and pancreatic lipases, blocking the absorption of dietary fats.7 For obesity management, orlistat should be dosed at 120 mg three times a day with a fatty meal. However, the side effects associated with orlistat are not well tolerated and include flatulence, fecal urgency, and steatorrhea, restricting its use in the pediatric population.1
Medications such as liraglutide, semaglutide, exenatide, and dulaglutide are classified as glucagon-like peptide-1 (GLP-1) receptor agonists that work by increasing glucose-dependent insulin secretion, slowing gastric emptying, and decreasing inappropriate glucagon secretion. In addition to their mechanism of action in the gut, these drugs target the areas of the brain regulating appetite control.7 This class of medication is indicated for treatment of type 2 diabetes and chronic weight management. Currently, semaglutide and liraglutide are both FDA approved for treatment of obesity in children aged 12 years and older.7,8 GLP-1 agonists are available as oral or SC injection formulations and may be dosed daily or weekly. For the treatment of obesity, liraglutide and semaglutide should be given as a SC injection at a maximum daily dosage of 3 mg and maximum weekly dosage of 2.4 mg, respectively.7
Setmelanotide, a melanocortin 4 (MC4) receptor agonist, is approved for use in children aged 6 years and older diagnosed with obesity due to Bardet-Biedl syndrome, proopiomelanocortin deficiency, proprotein convertase subtilisin/kexin type 1, or leptin receptor deficiency.1,7 MC4 receptors are found in the brain and control satiety, hunger, and energy expenditure. In children with the previously mentioned conditions, insufficient activation of MC4 leads to dysregulation of satiety and hunger. Setmelanotide reduces hunger, increases satiety, and increases energy expenditure by returning the MC4 receptor pathway to its normal state. Setmelanotide is available as an SC injection at a maximum daily dosage of 3 mg. Common adverse effects include, but are not limited to, hyperpigmentation, gastrointestinal upset, arthralgia, headache, dizziness, and trouble sleeping.
As one of the most commonly prescribed weight-loss medications among the adult population, phentermine is a sympathomimetic amine that works as an appetite suppressant secondary to its CNS effects and is approved for short-term (12-week) treatment of weight management in adolescents aged 16 years and older with a BMI ≥30 kg/m2 or a BMI ≥27 kg/m2 and ≥1 weight-associated comorbidity.1,7 Multiple doses and dosage forms exist and should be tailored to the patient at the lowest effective dose. Adverse effects of phentermine are dose-dependent and include increased blood pressure, dizziness, headache, tremor, and dry mouth.
Topiramate, indicated for migraine prevention and seizures with various off-label uses such as binge-eating disorder in adults, can cause appetite suppression through an unknown mechanism.1 One randomized, controlled trial compared the efficacy of topiramate 75 mg to placebo in treating adolescents aged 12 to 18 years with severe obesity. The study concluded no significant reduction in BMI in the placebo versus treatment group.9 Currently, topiramate is approved in children aged 2 years and older for seizure disorders and migraine prevention.7
As a combination product, phentermine with topiramate is approved for weight management in children aged 12 years and older with BMI ≥95th percentile or BMI ≥27 kg/m2 and ≥1 weight-associated comorbidity, in addition to lifestyle modifications.10 Phentermine with topiramate is available as an oral capsule and should be dose-adjusted based on reduction in BMI after 12 weeks on the maintenance dose (7.5 mg phentermine/46 mg topiramate).7
Lisdexamphetamine (Vyvanse) is currently approved for binge-eating disorder in adults aged 18 years and older and in children aged 6 years and older diagnosed with attention-deficit/hyperactivity disorder, though its mechanism of action in treating both indications is still unclear.7 Lisdexamphetamine’s most common adverse effect among adults and children alike remains decreased appetite. Though there is potential in using this drug in treating children with obesity, more studies are needed to provide efficacy and safety data.1
Metabolic and Bariatric Surgery
Within the past 2 to 3 decades, various observational studies have been conducted to determine the safety and efficacy of bariatric surgery versus other traditional treatment methods in treating adolescents with obesity.1 The data largely correspond to studies conducted in the adult population in which adolescents had a sustained reduction in BMI and improvements in their obesity-related comorbid conditions. Currently, the Roux-en-Y gastric bypass and vertical sleeve gastrectomy are among the most common bariatric surgeries completed in adolescents, with high rates of sustained BMI reduction. Complications from the surgery normally present in the early postoperative phase and include minor events such as nausea and diarrhea. The current criteria for bariatric surgery in adolescents are as follows: class 2 obesity, defined as BMI ≥35 kg/m2 or 120% of the 95th percentile for age and sex, plus clinically significant disease or class 3 obesity, defined as BMI ≥40 kg/m2 or 140% of the 95th percentile for age and sex. Current data pertaining to the safety and efficacy of bariatric surgery in children aged younger than 12 years are still minimal; thus, more studies are needed before making recommendations for bariatric surgery in this age group.
CONCLUSION
At present, pediatric obesity, a worldwide health crisis, has been met with limited assistance or resistance. Combating this epidemic is multifactorial, but education on healthy living should begin at the time of conception. The greatest tool in fighting the pandemic is prevention. If unsuccessful, multimodal treatment options are available depending on age. MI and IHBLT are the cornerstone of treatment, and both have been shown to have positive outcomes without any risk of adverse effects. Aside from aiding the patient, these therapeutic options can positively impact other family members as well. Pharmacologic agents can have a role in therapy; however, most of these agents are not FDA-approved for weight loss, with use based on a limited number of studies that had no long-term follow-up. The use of medications should be reserved for patients aged 12 years or older who are classified as obese and have obesity-related comorbid conditions.
The role of the pharmacist in combating pediatric obesity is currently undefined, but there is an opportunity for positive impact. Given the primary cause of pediatric overweight and obesity is unhealthy living, pharmacists could leverage their position in the community to provide education on nutrition and physical activity. Conversations with children and family members can be initiated at any point of the care experience, including during medication acquisition and counseling. Care should also be paid to medications that can contribute to weight gain and to suggesting to providers alternatives when available.
REFERENCES
1. Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640.
2. World Health Organization. Obesity and overweight. June 9, 2021. www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed May 17, 2023.
3. CDC. Childhood obesity facts. May 17, 2022. www.cdc.gov/obesity/data/childhood.html. Accessed May 17, 2023.
4. Harvard T.H. Chan School of Public Health. Defining childhood obesity. www.hsph.harvard.edu/obesity-prevention-source/obesity-definition/defining-childhood-obesity/. Accessed June 1, 2023.
5. CDC. Overweight & obesity: defining child BMI categories. Updated March 21, 2023. www.cdc.gov/obesity/basics/childhood-defining.html. Accessed June 1, 2023.
6. Sahoo K, Sahoo B, Choudhury AK, et al. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4(2):187-192.
7. Lexicomp Online, Lexi-Drugs Online. Waltham, MA: UpToDate, Inc.; May 15, 2023. https://online.lexi.com. Accessed May 21, 2023.
8. Collins L, Costella RA. Glucagon-like peptide-1 receptor agonists. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan. www.ncbi.nlm.nih.gov/books/NBK551568/.
9. Fox CK, Kaizer AM, Rudser KD, et al. Meal replacements followed by topiramate for the treatment of adolescent severe obesity: a pilot randomized controlled trial. Obesity (Silver Spring). 2016;24:2553-2561.
10. FDA. FDA approves treatment for chronic weight management in pediatric patients aged 12 years and older. June 27, 2022. www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-chronic-weight-management-pediatric-patients-aged-12-years-and-older.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





