US Pharm. 2023;48(3):45-48.
ABSTRACT: Heart failure with preserved ejection fraction (HFpEF) is a chronic disease that is increasing in prevalence and contributes to the overall incidence of heart failure. Sodium glucose cotransporter 2 inhibitors (SGLT2i) were introduced into guideline-directed medical therapy for heart failure with reduced ejection fraction and have been added to HFpEF guidelines based upon further trials. As a class-wide effect, SGLT2i have been proven to reduce the composite of hospitalizations and cardiovascular death in patients with HFpEF. These results provide evidence for the expansion of use of SGLT2i in the preserved ejection fraction subset of heart failure patients.
Heart failure (HF) is an increasingly prevalent disease state and is projected to affect more than 8 million people older than age 18 years by 2030.1 Mortality from HF is substantial, with current pharmacologic treatments aiming to target HF risk factors and improve survival; however, there are emerging data to suggest that these improvements in survival “could be leveling off over time.”1 The prevalence of HF with preserved ejection fraction (HFpEF), defined as an EF ≥50%, is rising and thought to be driving the overall increase of HF.1 Risk factors for HFpEF versus HF with reduced ejection fraction (HFrEF) include older age, female, hypertension, obesity, and anemia, with factors such as diabetes, coronary heart disease, smoking, and hypertension contributing to the overall HF risk.1 Guideline-directed treatment for HFrEF is well defined; however, the approach to HFpEF is less clear.
Mechanism of Action
Sodium glucose cotransporter 2 inhibitors (SGLT2i) were recently included in the treatment guidelines due to reduced hospitalizations for HF and cardiovascular (CV) mortality in patients with HFpEF.2 Aside from adequate blood pressure control, SGLT2i are the only direct therapy recognized in the guidelines to reduce mortality in HFpEF. These agents work by blocking the SGLT2 protein in the proximal tubule of the nephron, reducing the amount of reabsorbed glucose and sodium into the blood. This inhibition results in glycosuria and resulting natriuresis, ultimately lowering serum glucose concentrations.3 While the antihyperglycemic outcome achieved with these agents is important, several cardioprotective effects of SGLT2i have been proven in HF patients regardless of the presence of diabetes. These favorable effects include blood pressure lowering, natriuresis and diuresis, improved cardiac energy metabolism, prevention of inflammation, improved glucose control, and weight loss.4 Several potential mechanisms have been proposed, targeting HF risk factors to produce cardioprotective effects. This article examines the evidence of benefit for SGLT2i in patients with HFpEF.
The cardioprotective effects of SGLT2i in HFrEF have been proven regardless of diabetes status. The Cardiovascular and Renal Outcomes in Patients with Heart Failure (EMPEROR-Reduced) and the Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF) trials were elemental in expanding the use of SGLT2i in patients with HFrEF but without diabetes.5,6 Dapagliflozin and empagliflozin both demonstrated a significant reduction in CV death.5,6 In addition, empagliflozin was superior in reducing hospitalizations for HF, and dapagliflozin decreased rates of worsening HF.5,6 These results prompted inclusion of SGLT2i to the HFrEF guidelines as first-line agents with a class I recommendation in stage C or D HFrEF.2 This was a significant addition to HFrEF treatment; however, the results cannot be extrapolated to HFpEF patients due to the background characteristics of the trial populations.
As these agents were largely accepted in HFrEF treatment, recommendations for HFpEF still remained unclear. In an effort to evaluate SGLT2i in HFpEF populations, several randomized trials have thus been conducted to evaluate the effectiveness and overall benefit in this disease subset.
Randomized Trials
The relevant clinical trials evaluating evidence for SGLT2i in HFpEF are shown in TABLE 1. The EMPEROR-Preserved was the first landmark trial for SGLT2i in an HF population with a left ventricular ejection fraction (LVEF) of >40%.7 The randomized, double-blind, event-driven trial compared empagliflozin 10 mg to placebo, assessing the composite primary outcome of CV deaths or hospitalizations for HF. A total of 66.8% of patients had an EF ≥50%, and fewer than half (49%) of the trial participants had a diagnosis of diabetes. This trial proved the superiority of empagliflozin over placebo, as the trial group had a 21% lower relative risk of the composite primary outcome (6.9 vs. 8.7 events per 100 patient-years; hazard ratio [HR], 0.79; 95% CI 0.69-0.90; P <.001). This result was driven by a 29% lower risk of hospitalizations in the empagliflozin group (HR 0.71; 95% CI 0.60-0.83), as empagliflozin did not significantly reduce CV deaths alone. The benefits derived from empagliflozin in this population favor the use of SGLT2i in patients with HFpEF.7
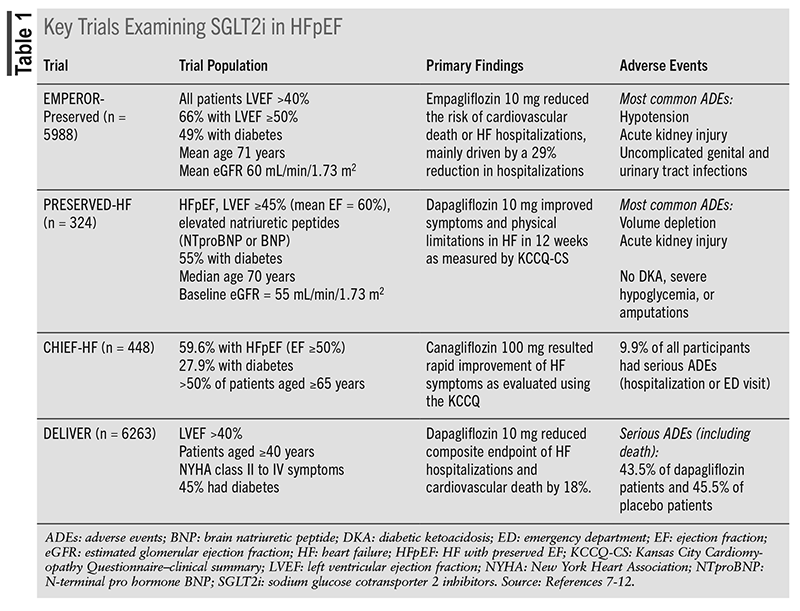
HF symptoms and physical limitations were evaluated in a HFpEF population randomized to dapagliflozin or placebo in the PRESERVED-HF trial.8 The trial used the Kansas City Cardiomyopathy Questionnaire Clinical Summary (KCCQ-CS) score measured at 12 weeks as the primary outcome of interest. This was assessed in a patient population with an overall mean EF of 60% and duration of HF around 3 years. The KCCQ is an international standard questionnaire completed by patients that enables practitioners to quantify the impact of HF on the patient’s health status (symptoms, function, and quality of life).9 The clinical summary score is a more specific average of physical limitation and total symptoms.9 Each domain is given a score of 0 to 100, with 0 being the worst and 100 being the best status, and a clinically meaningful improvement being an increase in ≥5 points.9 Dapagliflozin 10 mg improved overall symptoms in the KCCQ total symptom score (KCCQ-TSS) (effect size, 5.8 points; 95% CI 2.0-9.6, P = .003) and physical limitations (effect size for KCCQ-PL, 5.3 points (95% CI 0.7-10.0, P = .026). These results were consistent for all patients included in the trial, regardless of diabetes status.8
Canagliflozin similarly demonstrated a significant improvement in the quality of life of HF patients (both HFrEF and HFpEF), additionally illustrating that benefits can occur “as early as 2 weeks after initiation of therapy.”10 This was shown in the novel CHIEF-HF trial that utilized a decentralized, remote study design to assess the KCCQ-TSS at 12 weeks in patients randomized to canagliflozin 100 mg or placebo. Canagliflozin was favored by a mean improvement of 4.3 points on the KCCQ-TSS scale as compared with placebo (95% CI 0.8-7.8; P = .016), also improving the quality of life, physical limitation, and total daily step scores. Although no clinical outcomes were assessed, both PRESERVED-HF and CHIEF-HF prove the benefits of SGLT2i on patient lifestyle outcomes.10
The most recent trial is Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction (DELIVER), evaluating dapagliflozin 10 mg daily versus placebo.11 Patients were aged 40 years or older and were included if they had stabilized HF with an LVEF >40%, evidence of structural heart disease, an elevated natriuretic peptide level, and New York Heart Association (NYHA) functional class II-IV HF symptoms. The investigators included unique subgroups of patients such as those with recovered EF who previously had an EF <40% as long as it was >40% upon initiation of the trial. Patients were enrolled both as outpatients and during hospitalization for HF; however, the percentage of patients in each category was not specified. The mean LVEF in the empagliflozin group was 54.0±8.6% and was 54.3±8.9% in the placebo group. The breakdown of EFs was 33.7% of patients overall with an LVEF of 41% to 49%, 36% with an EF of 50% to 59%, and 30.1% with an EF ≥60%. Dapagliflozin 10 mg daily or placebo was studied in addition to usual HF therapy, including over 70% of patients on a loop diuretic or beta-blocker, and others on a renin-angiotensin-aldosterone system (RAAS) blocker or mineralocorticoid receptor antagonist (MRA). The primary outcome was a composite of worsening HF or CV death. Results were similar to the EMPEROR-Preserved trial, demonstrating a significant reduction of 18% in HF hospitalizations with dapagliflozin (HR 0.82; 95% CI 0.73-0.92, P <.001) and no significant difference in cardiovascular death. In the subgroup of patients with an EF >60%, results for the composite primary outcome were consistent with the findings overall. Previous trials suggested that the benefit of SGLT2i attenuates when the EF is >60%, thus DELIVER provides unique results that suggest these agents can be further extrapolated into those with higher EF and NYHA class II to IV symptoms.12 DELIVER strengthened the results of EMPEROR-Preserved and further supported the use of SGLT2i for the reduction of hospitalizations in HFpEF.11
A meta-analysis was conducted to evaluate SGLT2i on the primary outcome of a composite of time to CV death or first hospitalization for HF.12 EMPEROR-Preserved and DELIVER results were evaluated as the significant trials in HFpEF patients; the authors also included DAPA-HF, EMPEROR-Reduced, and SOLOIST-WHF to increase power to assess various cilnical endpoints. A majority of patients across all trials experienced NYHA class II symptoms, had a baseline median N-terminal (NT)-pro hormone BNP that ranged from 974 pg/mL to 1910 pg/mL, and minimum estimated glomerular filtration rate (eGFR) ranged from 20 to 30 mL/min/1.73m2. Results from EMPEROR-Preserved and DELIVER reduced the composite primary outcome, with results being consisent for a reduction in both time to cardiovascular death (HR 0.88; 95% CI 0.77-1.00) and first hospitalization for HF (HR 0.74; 95% CI 0.67-0.83). These data were generalizable across all patient backgrounds and subgroups. Importantly, the pooled results from EMPEROR-Preserved and DELIVER prove the benefits extend to patients with an LVEF >60%, a finding that has not been demonstrated with other pharmacologic options.12
Overall from the evaluation of the five trials in the meta-analysis, there was a significant reduction in the composite primary endpoint, with the “greatest benefit of SGLT2i to standard therapy in patients with HF [being] a 28% relative reduction in the risk of hospitalization for HF.”12 This produces a number needed to treat of 28 patients to prevent one CV death or hospitalization for HF event.12
Adverse Effects
Throughout each of the above trials, overall and serious adverse events were similar between trial medication and placebo groups. The prevalence of uncomplicated genital and urinary tract infections, as well as hypotension, was greater with empagliflozin in the EMPEROR-Preserved trial, which is consistent with previous findings from SGLT2i.7 Rates of diabetic ketoacidosis, hypoglycemia, and lower limb amputation were not increased with the use of SGLT2i.7-9 Importantly, no new adverse events were observed with SGLT2i agents beyond those previously found in HFrEF trials.
The results of the above SGLT2i trials have provoked recent changes to the AHA/ACC HF guidelines with regard to the management of HFpEF. Updates to the guidelines now give SGLT2i in HFpEF a 2a recommendation, the highest class of recommendation given to any agent for the treatment of HFpEF.2 Other guideline recommendations for HFpEF include diuretics for congestion and symptom improvement, as well as MRAs, angiotensin receptor blockers, and angiotensin receptor-neprilysin inhibitors to reduce hospitalizations in those “particularly with an LVEF on the lower end of the spectrum.”2 The addition of the above pharmacologic therapy recommendations is huge in the HFpEF subset, as previously practitioners were left with limited event-driven data in this population.
SGLT2i Use in Practice
Characteristics of patients in the trial populations should be considered when applying this evidence and recommendations to real-world use. In both EMPEROR-Preserved and PRESERVED-HF trials, SGLT2i agents were studied in addition to conventional HF-guideline–directed therapy. Most of the patients were taking RAAS agents, beta-blockers, loop diuretics, and statins at baseline, with approximately 30% of patients also taking an MRA upon initiation of the trials.7,8 This suggests that in practice SGLT2i should be used as concurrent therapy with other appropriate guideline-directed medical therapy for HF. Patients both with and without diabetes, as well as those with an eGFR <60 mL/min/m2, were all represented in the patient populations, thus showing the benefits of SGLT2i in HFpEF to be generalizable to this broad patient population.7,8 In the DELIVER trial, the addition of dapagliflozin was assessed in patients who were hospitalized for HF. While the results of the primary outcome were applicable to this patient population, the timing of initiation during the hospital stay was not defined, therefore requiring the need for future studies to evaluate the use of SGLT2i in hospitalized patients with HFpEF.
While the clinical trials showed no difference in adverse effects between SGLT2i and placebo, there are known side effects of SGLT2i. Side effects include hypovolemia, hypotension, acute kidney injury, urinary tract infections, genitourinary fungal infections, and few cases of ketoacidosis.13 The dose of SGLT2i when used for the indication of HF should also be considered when initiating therapy. Empagliflozin 10 mg is the only dose thus far that has been proven in both HFrEF and HFpEF trials to be safe and efficacious in providing the cardiac benefits.7 Dapagliflozin is also recommended as a 10-mg dose in HF patients, although it does not yet yield an FDA-labeled indication for HFpEF.13 The CHIEF-HF trial found the 100-mg daily dosage of canagliflozin improved HF symptoms in patients but did not assess benefits or safety outcomes at higher doses that can be used for other indications.10
Conclusion
SGLT2i have shown a reduction in the composite of CV death and hospitalizations for HF in patients with HFpEF. The EMPEROR-Preserved trial helped to gain initial utilization of these agents in patients with a preserved EF and inclusion in guidelines, while the DELIVER trial suggested that the benefits are generalizable to be a class-wide effect. SGLT2i present a desirable therapy option for an increasingly prevalent disease state that previously had few pharmacologic options. The benefits of SGLT2i provide a treatment option for HFpEF patients with minimal adverse effects, and overall they were well tolerated. Further studies in HFpEF patients are needed with power to show a reduction in CV death with these agents; however, they are generally safe and provide several advantages in HF, irrespective of LVEF.
REFERENCES
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145:e153-e639.
2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032.
3. Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. 2021;107:1032-1038.
4. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632-644.
5. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
6. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008.
7. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451-1461.
8. Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954-1960.
9. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469-476.
10. Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28:809-813.
11. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089-1098.
12. Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757-767.
13. Dapagliflozin. Lexi-Drugs. Hudson, OH: Lexicomp, 2022. http://online.lexi.com/. Updated August 25, 2022. Accessed August 30, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






