US Pharm. 2020:45(4)HS-1-HS-HS-6.
ABSTRACT: Candida auris (C auris) is an emerging multidrug-resistant yeast that presents a global health threat. Hospitalized patients and residents in long-term care facilities from several countries have fallen ill with C auris, with infections reported in over 30 countries. Thirty percent of reported cases were resistant to at least two antifungals. This pathogen can persist on surfaces and spread between patients in healthcare facilities. Primary infection-control measures should be followed to prevent transmission. Infections are diagnosed through either a blood culture or culture from other bodily fluids. Echinocandins are currently recommended as first-line therapy for the treatment of C auris infections in individuals aged 2 months and older. A novel antifungal agent currently in phase lll development has demonstrated potent fungicidal activity against C auris. Pharmacists can be involved in practicing antifungal stewardship and participating in the development of institutional guidelines and procedures for the management of C auris.
Candida auris (C auris) is a yeast that has been reported in healthcare facilities and has caused severe illness in hospitalized patients and residents in long-term care facilities. It was first identified in 2009 in Japan, with the earliest known strain dating to 1996 in South Korea. C auris is considered an emerging pathogen due to the increasing number of infections in more than 30 countries, with more than 900 clinical cases reported in the United States alone as of 2019. This fungus can spread through contaminated environmental surfaces or equipment, or from person to person.1-4
C auris can cause bloodstream infections, wound infections, and ear infections. It has been found in respiratory and urine specimens, but it is unknown if it causes lung or bladder infections. It does not respond to commonly used antifungal agents, making treatment difficult. Patients at risk for becoming infected include those hospitalized for long periods of time and those who have a central venous catheter, other lines or tubes, or who have previously received antibiotics or antifungal medications. More than one in three patients with C auris infection die. Ninety percent of isolates have been found to be resistant to at least one antifungal, while 30% of isolates have been found to be resistant to at least two antifungals.3-7
There is increasing concern about C auris because it is multidrug resistant, with some strains found to be resistant to all three classes of antifungals. Identification is difficult, and it can be misidentified. Outbreaks in healthcare settings have occurred; therefore, it is important to identify correctly to prevent spreading this potentially deadly pathogen.3
Due to these concerns, at the 2018 Council for State and Territorial Epidemiologists (CSTE) Annual Conference, reporting of C auris infections from healthcare facilities became mandated. Cases are classified according to definitions established by the CSTE. Clinical cases can be classified as confirmed, probable, or suspect, while colonization or screening cases can be confirmed or probable.8
The most up-to-date resource for information regarding C auris is the CDC. As of February 2020, the CDC reported a total of 1,018 cases of C auris. TABLE 1 identifies U.S. states that have reported confirmed clinical cases of C auris as of December 31, 2019.1
Screening and Diagnosis of C auris
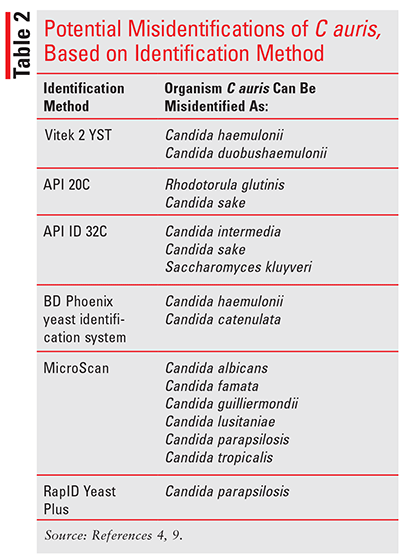
C auris is rare in the U.S., and people who become infected often suffer from other medical conditions, making it difficult to identify that they have become infected. Common symptoms include fever and chills that do not improve with antibiotic treatment. Infections are diagnosed through either a blood culture or culture from other bodily fluids. C auris can be confused with other yeasts, depending on the yeast-identification method used. TABLE 2 gives examples of potential misidentifications.4,9
The CDC recommends all yeast isolates obtained from sterile sites like the bloodstream and cerebrospinal fluid be identified to the species level. C auris from nonsterile body sites should be identified as well, because presence of C auris at any site can indicate colonization, which adds to risk for transmission and the need for infection-control precautions.10
Screening for C auris colonization is an important component of surveillance. Testing is available through the CDC’s AR Lab Network and is done free of charge. Commercial testing for screening is not available. Screening is done with a composite swab of the patient’s bilateral axilla and groin since data suggest that these sites are the most common loci of colonization.11
The following people should be considered for screening, because they are at high risk:
• Close healthcare contacts for those identified or colonized with C auris
• Patients who have stayed overnight at a healthcare facility outside the U.S. in the previous year, especially if it occurred in a country with documented
C auris.11
Infection-Control Recommendations
Primary infection-control measures should be followed and include:
• Practice hand hygiene
• Take transmission-based precautions
• Clean and disinfect the patient-care environment and reusable equipment
• Interfacility communications are important when the patient may need to be transferred to another facility
• Contacts of newly identified patients should be screened to identify C auris colonization
• Laboratory surveillance of clinical specimens is used to detect new cases
Personnel caring for a patient should follow standard hand hygiene. Alcohol-based hand sanitizer is effective against C auris. When diagnosed with C auris, contact precautions should be in place. Patients should be placed in a single room when possible. These patients should be prioritized, as they are at higher risk of pathogen transmission. In circumstances where it is not possible to separate an infected patient, strategies to reduce transmission include spatial separation by at least 3 feet, privacy curtains to limit contact, cleaning and disinfecting reusable equipment, and cleaning and disinfecting environmental surfaces on a more frequent schedule; personal protective equipment should be changed when moving between roommates.12
Treatment Options
Treatment of C auris infections is challenging due to the organism’s multidrug-resistant capabilities.13 All clinical C auris isolates should undergo antifungal susceptibility testing according to guidelines set forth by the Clinical & Laboratory Standards Institute (CLSI).14 The majority of C auris strains in the U.S. are resistant to fluconazole, but remain susceptible to echinocandins.14,15 More alarming are reports of pan-resistant C auris isolates worldwide, with three confirmed cases recently reported in New York.16 Pan-resistant C auris is defined as resistance to all three classes of commonly prescribed antifungals (fluconazole, amphotericin B, and echinocandins). Hence, consultation with an infectious disease specialist is essential when caring for patients with C auris infections.15
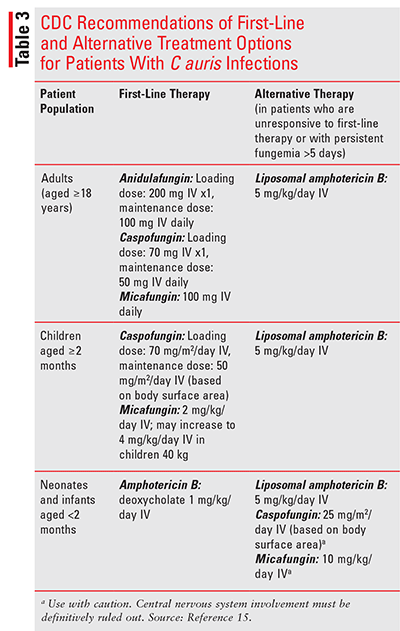
Echinocandins are currently recommended as first-line therapy for the treatment of C auris infections in individuals aged 2 months and older.15 The echinocandins act as noncompetitive inhibitors of an essential component of the fungal cell wall, beta-1,3-D-glucan synthase, leading to cell death.17 The FDA-approved agents in this class include anidulafungin, caspofungin, and micafungin. Agents in this class are available for IV administration only. Caspofungin and micafungin are approved for use in children, while anidulafungin is not. All of the echinocandins carry a warning for possible hepatic dysfunction, including hepatitis and hepatic failure.17-20 Hepatic dose adjustment with caspofungin is required in individuals aged 18 years and older with moderate liver impairment (Child-Pugh score 7 to 9). The hepatically adjusted dose for caspofungin is 70 mg IV loading dose, followed by 35 mg IV once daily. There are currently no dose-adjustment recommendations for adult patients with severe hepatic impairment (Child-Pugh score greater than 9) and in pediatric patients with any degree of hepatic impairment, owing to limited clinical data.19
In patients who are unresponsive to echinocandin therapy or who have persistent fungemia for more than 5 days, the CDC recommends switching to liposomal amphotericin B 5 mg/kg IV once daily.17 In a recently published case series involving nine patients with C auris infections in Brooklyn, New York, the authors encouraged the use of combination antifungal therapy with echinocandin plus liposomal amphotericin B in patients unresponsive to echinocandin monotherapy.21 This recommendation is due to the laboratory limitations of timely identification and susceptibility testing of C auris. Amphotericin B is associated with infusion-related reactions, particularly nausea, vom iting, chills, and rigors, renal insufficiency and electrolyte disturbances.22,23 These adverse effects are attenuated with lipid-based formulations of amphotericin B. However, daily monitoring of serum creatinine and electrolytes is warranted for all patients on amphotericin B therapy.
In children younger than age 2 months with C auris infections, the CDC recommends the use of amphotericin B as initial therapy. The formulation and dose recommended in neonates and infants younger than age 2 months is amphotericin B deoxycholate 1 mg/kg IV once daily. Liposomal amphotericin B may be considered at a dose of 5 mg/kg IV once daily in those who are unresponsive to conventional amphotericin B. Cautious use of echinocandins in this population may also be considered in rare, exceptional circumstances when central nervous system involvement has been definitively ruled out due to echinocandins’ poor distribution into the central nervous system. The CDC recommends the following doses when using echinocandins in children younger than age 2 months: caspofungin 25 mg/m2 (based on body surface area) IV once daily and micafungin 10 mg/kg IV once daily.15
TABLE 3 presents CDC recommendations for first-line and alternative treatment options for patients with C auris infections.15
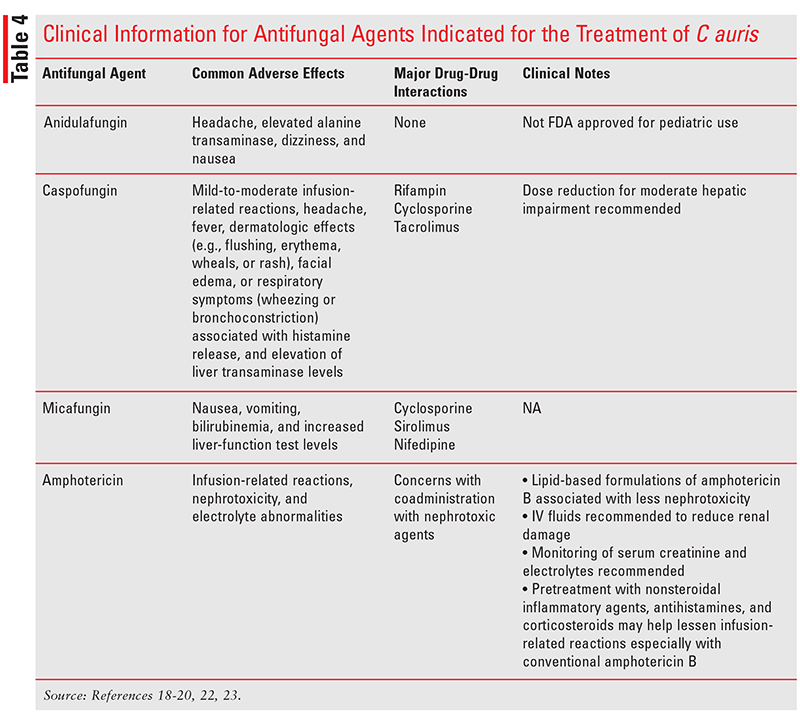
TABLE 4 summarizes pertinent clinical information for antifungal agents indicated for the treatment of C auris. 18-20,22,23
C auris Treatment Pipeline
Ibrexafungerp (formerly SCY-078), a novel antifungal agent currently in phase III development, has demonstrated potent fungicidal activity against C auris.24 This agent is the first in a novel class of structurally distinct glucan synthase inhibitors, triterpenoids, and is available in both IV and oral formulations.24 Data presented at ID Week 2019 (October 2-6, 2019, Washington, DC) revealed in vitro and in vivo activity of ibrexafungerp against C auris, including multidrug-resistant strains.25 Further clinical effectiveness was demonstrated in two patients with persistent C auris candemia enrolled in an ongoing, open-label study on the safety and effectiveness of ibrexafungerp in C auris infections (CARES Study, ClinicalTrials.gov, NCT03363841). The two patients failed conventional antifungal therapy and were successfully treated with oral ibrexafungerp.25 The CARES study is expected to enroll approximately 30 patients with an estimated completion date of May 15, 2021.25
Role of the Pharmacist
Pharmacists can be involved in practicing antifungal stewardship to help optimize patient outcomes with C auris and slow the emergence of this deadly fungus. Performing prospective audits and feedback on patients with C auris infections will ensure the appropriate selection of antifungal therapy and dosing. As drug experts, pharmacists can minimize adverse consequences when patients are placed on antifungal therapy by performing safety monitoring.
Pharmacists can also participate in developing institutional guidelines and procedures for the management of C auris, including proper identification of the organism and infection control and prevention measures. Since C auris is often misidentified in standard microbiology laboratories, pharmacists should be aware of the common misidentifications based on the laboratory instruments used at their institutions. A formal process should be established to send suspected C auris isolates to the CDC for proper identification and antifungal susceptibility testing. Once C auris is confirmed, it should be reported to local and state public health officials. Additionally, becoming familiar with primary infection-control measures will prevent the transmission of C auris in the facility.
Keeping up-to-date with the latest developments on C auris will allow pharmacists to be key educators on this important topic. Pharmacists can provide hospital-wide education through grand rounds, in-services, newsletters, and other types of educational forums to all disciplines across the healthcare facility on the most recent statistics and recommendations on C auris as they are released by the CDC.13 Moreover, pharmacists can provide education and counseling to patients and their families and caregivers on the treatment and management of C auris, as well as infection prevention and control measures.
REFERENCES
1. CDC. Tracking Candida auris. Updated February 6, 2020. www.cdc.gov/fungal/candida-auris/tracking-c-auris.html#world. Accessed February 6, 2020.
2. CDC. Information for infection preventionists. Updated December 21, 2018. www.cdc.gov/fungal/candida-auris/fact-sheets/cdc-message-infection-experts.html. Accessed February 6, 2020.
3. CDC. General information about Candida auris. Updated November 13, 2019. www.cdc.gov/fungal/candida-auris/candida-auris-qanda.html. Accessed February 6, 2020.
4. Jeffery-Smith A, Taori SK, Schelenz S, et al. Candida auris: a review of the literature. Clin Microbiol Rev. 2017;31:e00029-17.
5. Cortegiani A, Misseri G, Fasciana T, et al. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6:69.
6. CDC. Drug-resistant Candida auris. www.cdc.gov/drugresistance/pdf/threats-report/candida-auris-508.pdf. Accessed February 6, 2020.
7. CDC. Candida auris. Updated February 6, 2020. www.cdc.gov/fungal/candida-auris/index.html. Accessed February 6, 2020.
8. Council for State and Territorial Epidemiologists. (2018). Standardized case definition for Candida auris clinical and colonization/screening cases and national notification of C. auris case, clinical. Retrieved from https://cdn.ymaws.com/www.cste.org/resource/resmgr/ps/2018ps/18-ID-05_Dec2018_Update.pdf. Accessed March 26, 2020.
9. CDC. Identification of Candida auris. Updated October 16, 2019. www.cdc.gov/fungal/candida-auris/recommendations.html. Accessed March 11, 2020.
10. CDC. Surveillance for Candida auris. Updated December 21, 2018. www.cdc.gov/fungal/candida-auris/c-auris-surveillance.html. Accessed February 6, 2020.
11. CDC. Screening for Candida auris colonization. Updated February 6, 2020. www.cdc.gov/fungal/candida-auris/c-auris-screening.html. Accessed February 6, 2020.
12. CDC. Infection prevention and control for Candida auris. Updated January 28, 2020. www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html. Accessed February 6, 2020.
13. Lee Y, Bao H, Viramgama S. A rare fungus on the rise: Candida auris. Am J Health Syst Pharm. 2018;75(14):1013-1017.
14. CDC. Antifungal susceptibility testing and interpretation. Updated January 2, 2020. www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html. Accessed February 4, 2020.
15. CDC. Treatment and management of infections and colonization. Updated December 21, 2018. www.cdc.gov/fungal/candida-auris/c-auris-treatment.html. Accessed February 4, 2020.
16. Ostrowsky B, Greenko J, Adams E, et al. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:6-9.
17. Sucher AJ, Chahine EB, Balcer HE. Echinocandins: the newest class of antifungals. Ann Pharmacother. 2009;43(10):1647-1657.
18. Eraxis (anidulafungin) prescribing information. New York: Pfizer Inc.; August 2017.
19. Cancidas (caspofungin) prescribing information. Whitehouse Station, NJ: Merck Sharp & Dohme; February 2017.
20. Mycamine (micafungin) prescribing information. Deerfield, IL: Astellas Pharma; August 2016.
21. Park JY, Bradley N, Brooks S, et al. Management of patients with Candida auris fungemia at community hospital, Brooklyn, New York, USA, 2016–2018. Emerg Infect Dis. 2019;25(3):601-602.
22. Ambisome (amphotericin B liposome for injection) prescribing information. Northbrook, IL: Astellas Pharma; February 2019.
23. Amphotericin B intravenous injection prescribing information. Big Flats, NY: X-GEN Pharmaceuticals, Inc; April 2010.
24. Ibrexafungerp (formerly SCY-078): an innovative antifungal. Scynexis, Inc. Pipeline. www.scynexis.com/pipeline. Accessed February 4, 2020.
25. Barat S. Activity of ibrexafungerp (formerly SCY-078) against Candida auris: in vitro, in vivo and clinical case studies of candidemia. Poster #672 presented at IDWeek; October 2-6, 2019; Washington, DC. www.idweek.org. Accessed February 4, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.