US Pharm. 2023;48(10):43-45.
Ovarian cancer is a gynecologic malignancy that originates in the ovary (or ovaries) or involves related areas of the fallopian tubes and peritoneum. Often called a “silent killer,” ovarian cancer is the leading cause of death among women with gynecologic cancers. The symptoms of ovarian cancer are nonspecific, frequently resulting in delayed diagnosis and, consequently, greater rates of morbidity and mortality.1,2 Women with ovarian cancer typically present with vague, general symptoms such as abdominal or back pain, bloating, difficulty eating or feeling full quickly, or a change in bowel movements, which can be confused with symptoms of common gastrointestinal disorders.1,3 The incidence of ovarian cancer is higher in older women, with 50% of all cases occurring in those aged 63 years and older. Other risk factors for ovarian cancer include lifestyle factors, pregnancy characteristics, hormonal factors, family history, and genetic factors.1,4,5
Epidemiology and Etiology
Epidemiology: Ovarian cancer is the fifth leading cause of cancer deaths among women, and 236,511 U.S. women were estimated to have this disease in 2020.6,7 The highest rate of new ovarian cancer cases was in American Indian/Alaska Native women (11.3 per 100,000), followed by white (10.5 per 100,000), Hispanic (10 per 100,000), Asian/Pacific Islander (9.3 per 100,000), and black/African-American women (8.9 per 100,000).6 The American Cancer Society estimated that in 2023, 19,710 women would be newly diagnosed with ovarian cancer and 13,270 would die from it.7
Etiology: Approximately 85% to 90% of ovarian cancer cases are considered noninherited or arise sporadically.8 Inherited ovarian cancers are less common and occur as a result of gene mutations that are passed from generation to generation. Hereditary breast and ovarian cancer syndrome accounts for 65% to 85% of hereditary ovarian malignancies that are associated with germline mutations of breast cancer genes 1 and 2 (BRCA1, BRCA2). Additionally, BRCA1 has been shown to have a greater susceptibility and prevalence compared with other genes that have been linked to hereditary ovarian syndromes.9 Hereditary nonpolyposis colorectal cancer (Lynch syndrome) also is associated with germline mutations, increasing the lifetime risk of developing ovarian cancer to 12%.10 There are other gene mutations known to be associated with ovarian tumors, as well as unknown mutations as yet undetected by currently available tests.9
Diagnosis and Management
Diagnosis: Successful treatment of ovarian cancer is more likely when the disease is detected while still in the early stage (i.e., confined to one or both ovaries).11 Currently, no routine diagnostic procedure has been found to consistently reduce the mortality associated with ovarian cancer.12 The invasiveness of diagnostic surgery carries the risk of harm related to false-positive test results, and there are other screening methods for early detection of ovarian cancer.12 Transvaginal ultrasonography is used to detect changes in ovarian cells’ size and morphology before any symptoms of ovarian cancer emerge; however, this screening method lacks specificity and the ability to detect peritoneal or ovarian cancer in patients without morphologic abnormalities of the ovary. The cancer antigen 125 (CA-125) test has been used to detect increased serum levels of the tumor marker CA-125 in women with ovarian cancer, but although CA-125 has been studied extensively, it is nonspecific and therefore not recommended for routine screening.12-14 Genetic testing should be considered for patients whose family history puts them at increased risk for ovarian cancer.15
According to the U.S. Preventive Services Task Force, ovarian-cancer screening is not recommended in asymptomatic women who are not at high risk for hereditary cancer syndromes.16,17 The nonspecific nature of the clinical symptoms of ovarian cancer adds to the challenge of detecting this disease and initiating optimal treatment plans to lessen the likelihood of mortality.12,16,17
Management: Standard treatment for newly diagnosed ovarian cancer generally involves surgical staging and debulking surgery, systemic platinum-based chemotherapy, or a combination of both.18 Because these options are limited, advanced therapies have been studied to improve treatment outcomes and overall survival. An emerging class of drugs, the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors, has shown promise for the treatment of ovarian cancer.19
The PARP inhibitor niraparib (Zejula) is FDA approved as first-line maintenance treatment for patients diagnosed with advanced epithelial ovarian, fallopian-tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy.20 Niraparib is also indicated for maintenance treatment in adults with deleterious or suspected deleterious germline BRCA-mutated recurrent epithelial ovarian, fallopian-tube, or primary peritoneal cancer.20,21 Other PARP inhibitors indicated for ovarian cancer include olaparib (Lynparza) and rucaparib (Rubraca).19 Veliparib is currently undergoing clinical investigation as an addition to first-line chemotherapy prior to maintenance in patients with high-grade serous ovarian carcinoma.22
MyChoice CDx Test
The MyChoice CDx companion diagnostic, a single-site assay developed by Myriad Genetics, is the only comprehensive tumor laboratory test that specifically identifies homologous recombination deficiency (HRD) status in patients diagnosed with ovarian cancer.23,24 HRD is defined as a deleterious or suspected deleterious BRCA mutation and/or high genomic instability score (GIS).23,24 The HRD triggers a loss of heterozygosity; telomeric allelic imbalance, which indicates defective DNA repair and sensitivity to DNA-damaging agents; and large-scale state transition, which refers to the number of chromosomal breaks in a tumor genomic profile.25,26 MyChoice CDx is also an FDA-approved companion diagnostic for niraparib and olaparib. A positive test result determines the patient’s eligibility for PARP inhibitor treatment in accordance with the product’s indications for use.23,24
The sample collection kit contains a slide container, tumor-block container, collection instructions, mailing instructions, and ice pack. Although the collection kit provides two options for collection, tumor-block samples are preferred over slides whenever possible. The clinician must collect a small amount of cancer tissue from a patient’s tumor using the MyChoice CDx tumor-specimen collection and transportation kit.23 Once the specimen is obtained, it is mailed to Myriad Genetics, where it undergoes DNA-sequence analysis. The isolated DNA from the formalin-fixed, paraffin-embedded tumor-tissue specimens is evaluated for changes in BRCA1 and BRCA2 genes and determination of GIS. Currently, there are no contraindications or major warnings and precautions for MyChoice CDx use.23
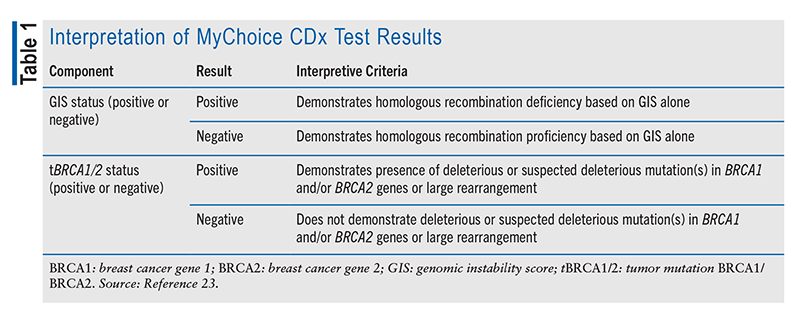
The interpretive criteria for MyChoice CDx results are based on the observation of variations in DNA sequencing behavior and the cell’s ability to repair its original DNA structure.23 It may take up to 14 days to receive results. The test results are ultimately pivotal for developing an optimal treatment plan for the patient.23 TABLE 1 summarizes how the results are interpreted after DNA analysis.
Efficacy
The efficacy of the MyChoice CDx test was assessed in the QUADRA clinical trial and a clinical bridging study.
QUADRA Study: MyChoice CDx was used for tumor testing in a multicenter, open-label, single-arm, phase II clinical trial conducted in the U.S. and Canada. The purpose of this study was to assess the safety and efficacy of niraparib in women with recurrent high-grade serous ovarian cancer who were previously treated with at least three previous chemotherapy regimens and were HRD-positive (according to the MyChoice clinical trial assay [CTA]).23,27 Based on the CTA results, 463 patients were enrolled and received treatment with niraparib. Niraparib demonstrated clinical response in patients with difficult-to-treat ovarian cancer and benefited patients with or without BRCA1/2 mutation. These study results expanded niraparib’s indication to include women with HRD-positive ovarian cancer.27
Clinical Bridging Study: In the bridging study, MyChoice CDx was used to retrospectively assess all tissue samples from participants in the QUADRA study. The MyChoice CDx test was compared with the MyChoice CTA to establish clinical validity in identifying a patient’s HRD status. As a result of the retrospective study, the concordance between the MyChoice CTA and MyChoice CDx yielded a positive percent agreement of 100% for all valid BRCA1/2 sequence variant calls and a negative percent agreement of 99.99% for all valid BRCA1/2 sequence nonvariant calls.23 Overall, the QUADRA and bridging studies demonstrated the concordance of patients’ HRD status with MyChoice CDx, supporting its utility in determining the eligibility of ovarian cancer patients for treatment with niraparib and olaparib.23,27,28
Conclusion
MyChoice CDx is a companion diagnostic used to identify ovarian cancer patients with a positive HRD status who may be eligible for treatment with the PARP inhibitors niraparib and olaparib. Use of this test increases the available treatment options and helps clinicians make clinically sound decisions that improve outcomes in patients in early and late stages of ovarian cancer. Refer to Myriad Genetics’ product website at https://myriad.com/genetic-tests/mychoicecdx-tumor-test/ for more information about MyChoice CDx.
REFERENCES
1. CDC. Ovarian cancer. www.cdc.gov/cancer/ovarian/index.htm. Accessed September 20, 2023.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
3. Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2015;131(Suppl 2):s111-s122.
4. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287-299.
5. Rooth C. Ovarian cancer: risk factors, treatment and management. Br J Nurs. 2013;22(17):s23-s30.
6. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 24, 2023.
7. American Cancer Society. Key statistics for ovarian cancer. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed May 4, 2023.
8. Gennatas C. The genetics of ovarian cancer. In: Díaz-Padilla I, ed. Ovarian Cancer: A Clinical and Translational Update. London, England: IntechOpen; 2013:135-158.
9. Toss A, Tomasello C, Razzaboni E, et al. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed Res Int. 2015;2015:341723.
10. Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105(3):569-574.
11. Das PM, Bast RC Jr. Early detection of ovarian cancer. Biomark Med. 2008;2(3):291-303.
12. Committee on Gynecologic Practice Society of Gynecologic Oncology. Committee opinion no. 716: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstet Gynecol. 2017;130(3):e146-e149.
13. van Nagell JR Jr, Hoff JT. Transvaginal ultrasonography in ovarian cancer screening: current perspectives. Int J Womens Health. 2013;6:25-33.
14. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131(5):909-927.
15. Committee on Practice Bulletins–Gynecology, Committee on Genetics, Society of Gynecologic Oncology. Practice bulletin no 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110-e126.
16. Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(6):595-606.
17. US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(6):588-594.
18. Armstrong DK, Alvarez RD, Backes FJ, et al. NCCN Guidelines® insights: ovarian cancer, version 3.2022. J Natl Compr Canc Netw. 2022;20(9):972-980.
19. Kurnit KC, Fleming GF, Lengyel E. Updates and new options in advanced epithelial ovarian cancer treatment. Obstet Gynecol. 2021;137(1):108-121.
20. Zejula (niraparib) product information. Durham, NC: GlaxoSmithKline; April 2023.
21. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402.
22. Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403-2415.
23. FDA. myChoice CDx® technical information. www.accessdata.fda.gov/cdrh_docs/pdf19/P190014S003C.pdf. Accessed May 24, 2023.
24. Myriad Genetics, Inc. MyChoice CDx Myriad HRD companion diagnostic test. https://myriad.com/genetic-tests/mychoicecdx-tumor-test. Accessed September 20, 2023.
25. Frey MK, Pothuri B. Homologous recombination deficiency (HRD) testing in ovarian cancer clinical practice: a review of the literature. Gynecol Oncol Res Pract. 2017;4:4.
26. Takaya H, Nakai H, Takamatsu S, et al. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep. 2020;10(1):2757.
27. Moore K, Secord AA, Geller M, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636-648.
28. Arora S, Balasubramaniam S, Zhang H, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist. 2021;26(1):e164-e172.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





