US Pharm. 2023;48(10):17-26.
New molecular entities (NMEs), as defined by the FDA, are new drug products containing as their active ingredient a chemical substance marketed for the first time in the United States. The following descriptions of NMEs approved in 2022–2023 (TABLE 1) detail the basic clinical and pharmacologic profiles of each new drug, as well as key precautions and warnings. Also included are brief summaries of selected pharmacokinetic, adverse-reaction, drug-interaction, and dosing data submitted to the FDA in support of the manufacturer’s New Drug Application. This review is intended to be objective rather than evaluative in content. The information for each NME was obtained primarily from sources published prior to FDA approval. Experience clearly demonstrates that many aspects of an NME’s therapeutic profile are not detected in premarketing clinical studies and emerge after the drug is used in the broader population. For example, previously unreported adverse reactions become apparent for some NMEs within several years of their marketing. Some NMEs may eventually acquire at least one black box warning for serious adverse drug reactions or are withdrawn from the market for safety reasons not recognized at the time of approval. Therefore, while this review offers an introduction to some new drugs, it is essential that practitioners be aware of changes in their therapeutic profiles as reported in the pharmaceutical literature and by patients.
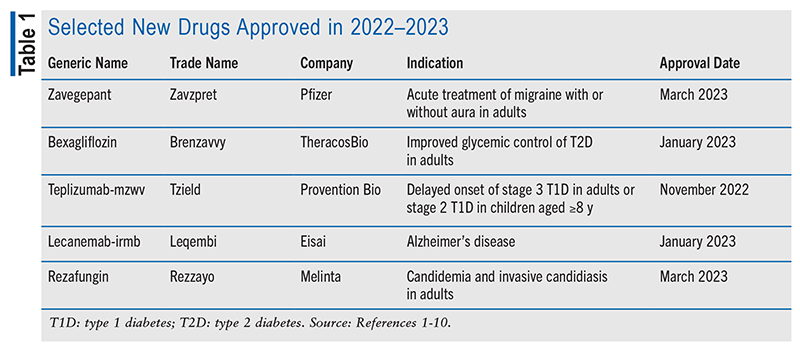
Zavegepant (Zavzpret, Pfizer)
Indication and Clinical Profile1,2: Zavegepant is indicated for acute treatment of migraine with or without aura in adults. Migraine pain occurs when nerve fibers in the walls of blood vessels inside the meninges are activated. The introduction of calcitonin gene-related peptide (CGRP) antagonists was a significant advance in migraine treatment. Zavegepant, the first CGRP antagonist formulated as a nasal spray, is an option for migraineurs who cannot take oral medications because of nausea or vomiting.
The safety and efficacy of zavegepant for acute migraine treatment were assessed in two randomized, double-blind, placebo-controlled trials. Zavegepant was statistically superior to placebo on the coprimary endpoints of pain freedom (reduction of moderate or severe headache pain to no headache pain) and freedom from patient’s most troublesome symptom at 2 hours post dose (absence of most troublesome self-identified symptom). In the phase III study, 1,405 subjects were randomized to receive a single 10-mg dose of zavegepant or placebo. Trial participants historically experienced two to eight moderate or severe migraine attacks per month, with untreated attacks lasting a mean of 30.8 hours. Zavegepant was statistically superior to placebo on 13 of 17 prespecified secondary outcome measures, including early time point endpoints (e.g., 15- and 30-minute pain relief, return to normal function at 30 minutes), return to normal function at 2 hours, and durable efficacy endpoints (e.g., 2- to 24-hour and 2- to 48-hour sustained pain freedom and sustained pain relief). The difference between zavegepant and placebo in return to normal function at the 15-minute postdose endpoint was not significant.
Pharmacology and Pharmacokinetics1,2: Zavegepant (FIGURE 1) is a dihydroquinolone CGRP receptor antagonist. The pathophysiology of migraine has not been fully elucidated, but the release of vasoactive substances such as CGRP, neurokinin A, nitric oxide, and substance P appears to contribute to the increased vasodilation and neuronal excitability that cause pain transmission in the trigeminal system. CGRP receptor antagonists such as zavegepant inhibit vasodilation mechanisms and desensitize neuronal circuits, reducing migraine pain.
Peak plasma concentrations of zavegepant are attained approximately 30 minutes after administration of a single 10-mg dose of nasal spray. Intranasal zavegepant’s mean apparent volume of distribution is approximately 1,774 L, and its plasma protein binding is 90%. Zavegepant undergoes a small degree of metabolism by CYP3A4 and CYP2D6. The effective half-life following a 10-mg dose of nasal spray is 6.55 hours. Zavegepant is excreted mostly via the biliary/fecal route (80%) in unmetabolized form; therefore, mild-to-moderate hepatic or renal impairment is not anticipated to significantly alter plasma levels.
Adverse Reactions and Drug Interactions1,2: The most common adverse reactions reported for zavegepant (>2% of patients and greater than placebo) included taste disorders (dysgeusia, ageusia), nausea, nasal discomfort, and vomiting. Zavegepant is contraindicated in patients with a history of hypersensitivity to the drug or any of its components. Hypersensitivity reactions, including facial swelling and urticaria, have occurred in clinical trials. Data on the developmental risks associated with zavegepant use in pregnant women are inadequate; however, no adverse developmental effects were observed in pregnant animals following SC administration of doses with plasma exposures higher than those used clinically. There are no data on the presence of zavegepant or its metabolites in human milk, or on its effects on breastfed infants or milk production. The health benefits of breastfeeding should be considered alongside the mother’s clinical need for, and potential benefits of, zavegepant use.
Concomitant administration of zavegepant with intranasal decongestants may decrease absorption and should be avoided. If concomitant use is unavoidable, intranasal decongestants should be administered >1 hour after zavegepant. Concomitant administration of zavegepant with organic anion transporting polypeptide 1B3 (OATP1B3) inhibitors or sodium taurocholate cotransporting polypeptide (NTCP) transporters results in a significant increase in zavegepant exposure and therefore should be avoided. Also, concomitant administration of zavegepant with inducers of OATP1B3 or NTCP transporters may result in a decrease in drug exposure and thus concurrent administration with other drugs that induce OATP1B3 or NTCP transporters should be avoided.
Dosage and Administration1,2: Zavegepant is supplied as an intranasal device, with each unit-dose device delivering a single nasal spray containing 10 mg of zavegepant. The recommended dosage is a single 10-mg spray into one nostril as needed. The maximum dosage to be administered in a 24-hour period is 10 mg (1 spray). The safety of treating more than eight migraines in a 30-day period has not been established. No dose adjustment is necessary in patients with mild (Child-Pugh class A) or moderate hepatic impairment (Child-Pugh class B) or in patients with estimated creatine clearance (CrCl) >30 mL/minute; however, use should be avoided in patients with severe hepatic impairment and those with CrCl <30 mL/minute.
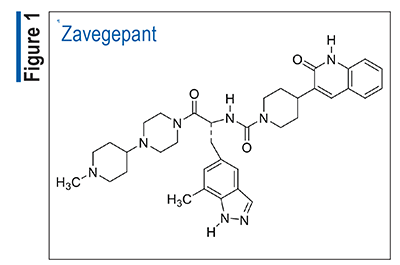
Bexagliflozin (Brenzavvy, TheracosBio)
Indication and Clinical Profile3,4: Bexagliflozin is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes (T2D). FDA approval was based on results of >20 clinical trials with a combined enrollment of >5,000 adults with T2D. In phase III studies, bexagliflozin significantly reduced A1C and fasting blood sugar after 24 weeks whether as monotherapy, in combination with metformin, or as an add-on to standard-of-care treatments including sulfonylureas, insulin, dipeptidyl peptidase 4 inhibitors, or combinations of these agents. Modest reductions in weight and systolic blood pressure also were reported.
Pharmacology and Pharmacokinetics3,4: Bexagliflozin (FIGURE 2), an oral agent, is an inhibitor of sodium-glucose cotransporter 2 (SGLT2), the proximal renal transporter responsible for reabsorption of most of the glucose from the glomerular filtrate. SGLT2 inhibition reduces renal reabsorption of filtered glucose, thereby increasing urinary glucose excretion and reducing plasma glucose levels.
Following oral administration, peak plasma concentrations of bexagliflozin are reached within 2 to 4 hours, but this may be delayed if bexagliflozin is taken after a meal or by medications that slow gastric emptying. Plasma maximum concentration of drug and AUC increase in a dose-proportional manner up to 90 mg. Bexagliflozin is approximately 93% bound to plasma protein and has an apparent volume of distribution of 262 L. It is metabolized primarily by UDP-glucuronosyltransferase (UGT) 1A9 glucuronidation and to a lesser extent by CYP3A. The most abundant metabolite in plasma is 3´-O-glucuronide. None of the metabolites appear to contribute to the glucose-lowering effects. The apparent terminal elimination half-life of bexagliflozin is about 12 hours. The drug is excreted nearly equally by the liver (51%) and the kidney (40%), largely as the 3´-O-glucuronide.
Adverse Reactions and Drug Interactions3,4: The most common adverse reactions reported for bexagliflozin (incidence >5%) included female genital mycotic infection, urinary tract infection (UTI), and increased urination; some UTIs were serious (including urosepsis and pyelonephritis) and required hospitalization. Ketoacidosis was also reported in clinical trials; if ketoacidosis is suspected, the drug should be discontinued and prompt treatment initiated. Compared with placebo patients, bexagliflozin patients had an increased incidence of lower-limb amputations. Necrotizing infections of the perineum (Fournier’s gangrene) have occurred; patients who develop pain or tenderness, redness, or swelling in the genital or perineal areas, along with fever, should should be assessed promptly. Bexagliflozin can cause intravascular volume contraction, which may manifest as symptomatic hypotension or acute transient changes in creatinine; therefore, renal function and signs and symptoms of volume depletion should be monitored. Because animal data demonstrated adverse renal effects, bexagliflozin use is not recommended during the second and third trimesters of pregnancy or while breastfeeding.
Bexagliflozin may increase the risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue. To minimize the risk, a lower dose of insulin or insulin secretagogue may be required. There are no clinically meaningful changes in bexagliflozin exposure when taken with metformin. Based on in vitro studies, bexagliflozin is not expected to inhibit or induce CYP450 isoenzymes (1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4) at clinically relevant plasma concentrations; however, UGT enzyme inducers may significantly reduce exposure to bexagliflozin, leading to decreased efficacy. Although it is a substrate for P-glycoprotein, bexagliflozin does not appear to inhibit drug transporters including breast cancer resistance protein, bile salt export pump, organic anion transporting polypeptides (OATP1B1, OATP1B3), anion transporters (OAT1, OAT3), organic cation transporters (OCT1, OCT2), and multidrug and toxin extrusion transporters (MATE1, MATE2-K) at clinically relevant plasma concentrations.
Dosage and Administration3,4: Bexagliflozin is supplied as 20-mg film-coated caplets for oral administration. The recommended dosage is 20 mg orally once daily in the morning, with or without food. Tablets should not be crushed or chewed. If a dose is missed, it should be taken as soon as possible, but the next dose should not be doubled. Renal function should be assessed before initiating therapy and this drug is not recommended if epidermal growth factor receptor is less than 30 mL/min/1.73m or patients are on dialysis. Volume depletion should be corrected before bexagliflozin is initiated. No dose adjustments are recommended for patients with mild-to-moderate hepatic impairment; however, bexagliflozin has not been studied in patients with severe hepatic impairment, and it is not recommended for these patients.
Teplizumab-mzwv (Tzield, Provention Bio)
Indication and Clinical Profile5,6: Teplizumab-mzwv is indicated to delay the onset of stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged >8 years with stage 2 T1D. The safety and efficacy of teplizumab-mzwv were evaluated in a randomized, double-blind, event-driven, placebo-controlled trial in 76 patients aged 8 years to 49 years with stage 2 T1D. Patients randomly received teplizumab-mzwv or placebo once daily via IV infusion for 14 days. The primary efficacy measure was the time from randomization to development of stage 3 T1D diagnosis (presence of >2 T1D-related autoantibodies and dysglycemia). Over a median follow-up of 51 months, 45% of the 44 patients who received teplizumab-mzwv were later diagnosed with stage 3 T1D, compared with 72% of the 32 patients given placebo. The median time from randomization to stage 3 T1D diagnosis was 50 months for teplizumab-mzwv patients and 25 months for placebo patients, representing a statistically significant delay in developing stage 3 T1D.
Pharmacology and Pharmacokinetics5,6: Teplizumab-mzwv is a cluster of differentiation (CD) 3–directed antibody that functions as a partial agonist, deactivating pancreatic beta cell autoreactive T lymphocytes. It also produces an increase in the proportions of regulatory T cells and exhausted CD8+ T cells in peripheral blood. Through these actions, teplizumab-mzwv prevents the immune-cell attack on insulin-producing pancreatic cells and delays the progression of T1D to stage 3 disease.
The central volume of distribution of teplizumab-mzwv is estimated to be 2.3 L, and the drug shows saturable protein binding and elimination. Steady-state concentrations are not achieved during the 14-day therapy course. The mean terminal elimination half-life and clearance of teplizumab-mzwv are 4.5 days and 2.7 L/day, respectively, in a 60-kg subject. Teplizumab-mzwv is expected to be metabolized into small peptides by catabolic pathways. No clinically significant differences in the pharmacokinetics of teplizumab-mzwv were observed based on age, sex, or race.
Adverse Reactions and Drug Interactions5,6: The most common adverse reactions reported for teplizumab-mzwv (>10%) included lymphopenia, rash, leukopenia, and headache. Teplizumab-mzwv carries warnings and precautions, including premedicating and monitoring for symptoms of cytokine release syndrome (CRS); risk of serious infections; decreased lymphocyte levels; risk of hypersensitivity reactions; the need to administer all age-appropriate vaccinations prior to teplizumab-mzwv initiation; and avoiding concurrent use of live, inactivated, and messenger RNA vaccines. Liver-enzyme (alanine aminotransferase, aspartate aminotransferase) monitoring is recommended to identify possible CRS. Also, monitoring of WBC counts during the treatment period is recommended and drug discontinuation is advised if prolonged severe lymphopenia (<500 cells/mcL for >1 week) develops.
Available case reports from clinical trials of teplizumab-mzwv are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Although there are no data on the following for teplizumab-mzwv, monoclonal antibodies can be actively transported across the placenta and may cause immunosuppression in the utero-exposed infant. To minimize fetal exposure, teplizumab-mzwv use should be avoided during pregnancy and >30 days (six half-lives) prior to a planned pregnancy. The developmental and health benefits of breastfeeding should be considered alongside the mother’s clinical need for teplizumab-mzwv and any potential adverse effects on the breastfed child.
Dosage and Administration5,6: Teplizumab-mzwv is supplied as a 2 mg/2 mL (1 mg/mL) clear solution in a single-dose vial for IV administration; it must be diluted in 0.9% Sodium Chloride Injection before use. Prior to administration, stage 2 T1D should be confirmed by documenting at least two positive pancreatic islet autoantibodies, in patients who have dysglycemia without overt hyperglycemia, using an oral glucose tolerance test (OGTT) or alternative method—if appropriate—and OGTT is not available. It should also be ascertained that the patient’s clinical history does not suggest type 2 diabetes. CBC and liver-enzyme tests should be obtained prior to therapy initiation. Teplizumab-mzwv should be administered by IV infusion over a minimum of 30 minutes, once daily for 14 days, at a dosing regimen of 65 mcg/m2 on day 1, 125 mcg/m2 on day 2, 250 mcg/m2 on day 3, 500 mcg/m2 on day 4, and 1,030 mcg/m2 on days 5 through 14.
Lecanemab-irmb (Leqembi, Eisai)
Indication and Clinical Profile7,8: Lecanemab-irmb was granted accelerated approval for the treatment of Alzheimer’s disease (AD), a progressive, irreversible brain disorder that destroys thinking and memory and, over time, the ability to perform everyday tasks. Although the causes are not completely understood, AD is characterized by brain changes, including amyloid-beta plaques and neurofibrillary tangles, that lead to the loss of neurons and their connections. Lecanemab-irmb is second in a new category of drugs that target the amyloid plaques associated with AD.
The efficacy of lecanemab-irmb was evaluated in a double-blind, placebo-controlled, parallel-group, dose-finding trial involving 856 AD patients with mild cognitive impairment or mild dementia disease stage and a confirmed presence of amyloid-beta pathology via positron emission tomography (PET) imaging. Patients were randomized to treatment with one of five dosages of lecanemab-irmb or placebo. Compared with placebo patients, those receiving lecanemab-irmb 10 mg/kg every 2 weeks had significant dose- and time-dependent reductions of amyloid-beta plaque at week 79, as documented by PET imaging. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
Pharmacology and Pharmacokinetics7,8: Lecanemab-irmb, a humanized immunoglobulin gamma (IgG) 1 monoclonal antibody, targets and reduces aggregated soluble and insoluble forms of amyloid beta. The accumulation of amyloid-beta plaques in the brain is a defining pathophysiological feature of AD.
The mean value for central volume of distribution at steady state after IV infusion is 3.22 L. Lecanemab-irmb is metabolized by proteolytic enzymes in the same fashion as endogenous IgGs. The clearance is 0.434 L/day, and the terminal half-life is 5 to 7 days. Differences in sex, body weight, and albumin do not appear to result in clinically significant alterations in kinetics. No clinical studies have been conducted to evaluate the pharmacokinetics of lecanemab-irmb in patients with renal or hepatic impairment, but impacts on drug clearance are not anticipated.
Adverse Reactions and Drug Interactions7,8: The most common adverse reactions reported for lecanemab-irmb (>10% incidence and less than placebo) included infusion-related reactions, headache, and amyloid-related imaging abnormalities (ARIA). Some patients with ARIA experienced headache, confusion, dizziness, vision changes, nausea, and seizure. Serious, life-threatening events associated with ARIA can occur, although this is rare. Lecanemab-irmb carries a black box warning for infusion-related reactions, including flulike symptoms, nausea, vomiting, and blood-pressure changes; these may be reduced by premedication with antihistamines, nonsteroidal anti-inflammatory drugs, or corticosteroids. When infusion reactions occur, the infusion rate may be reduced or the infusion may be discontinued, with appropriate therapy administered as clinically indicated. There are no safety or efficacy data on initiating treatment at earlier or later disease stages than were studied.
Dosage and Administration7,8: Lecanemab-irmb is supplied as solutions of 500 mg/5 mL (100 mg/mL) and 200 mg/2 mL (100 mg/mL) in single-dose vials for IV infusion. The recommended dosage is 10 mg/kg, so the solution must be diluted and administered as an IV infusion over approximately 1 hour, once every 2 weeks. If an infusion is missed, the next dose should be administered as soon as possible. Prior to treatment initiation, a recent (<1 year) brain MRI should be obtained to detect preexisting ARIA; MRI should also be done before the 5th, 7th, and 14th infusions. If radiographically observed ARIA occur, treatment may be modified based on the type and severity of the abnormality as well as the presence of symptoms.
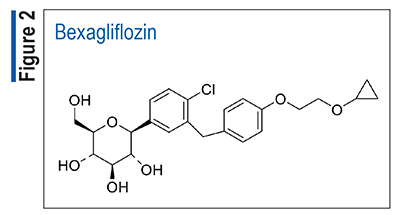
Rezafungin (Rezzayo, Melinta)
Indication and Clinical Profile9,10: Rezafungin, a weekly injectable echinocandin, is indicated for treatment of candidemia and invasive candidiasis in adults with limited or no alternative options. The Infectious Diseases Society of America recommends echinocandins as first-line therapy, except when an infection affects the central nervous system, eyes, or urinary tract. Antifungals other than echinocandins are considered alternatives in the case of drug intolerance, for drug-resistant pathogens, or as adjunctive therapy for refractory cases.
Approval of rezafungin was based on data from the ReSTORE and STRIVE clinical trials. ReSTORE was a global, double-blind, randomized study of patients aged >18 years with systemic signs and mycologic confirmation of candidemia or invasive candidiasis. Patients were assigned 1:1 to receive IV rezafungin (400 mg on week 1, followed by 200 mg once weekly, for a total of 2-4 doses) or IV caspofungin (70-mg loading dose on day 1, followed by 50 mg daily for <4 weeks). The primary endpoints were global cure (i.e., clinical cure, radiologic cure, and mycologic eradication) at day 14 for the European Medical Agency and 30-day all-cause mortality for the FDA, both with a target noninferiority margin of 20%, assessed in the modified intent-to-treat population. Fifty-five (59%) of 93 rezafungin patients and 57 (61%) of 94 caspofungin patients had a global cure at day 14; 22 (24%) rezafungin patients and 20 (21%) caspofungin patients had died or had unknown survival status at day 30. Comparable results were obtained in the STRIVE study, which compared rezafungin with caspofungin using similar dosing. Rezafungin has not been studied in patients with endocarditis, osteomyelitis, or meningitis due to Candida.
Pharmacology and Pharmacokinetics9,10: Rezafungin (FIGURE 3) is a semisynthetic lipopeptide synthesized from a fermentation product of Aspergillus nidulans. This echinocandin inhibits 1,3-beta-D-glucan, an essential component of the cell walls of many fungi, and inhibition of its synthesis results in concentration-dependent fungicidal activity against Candida species.
In patients given an initial 400-mg loading dose of rezafungin followed by a 200-mg dose once weekly, maximum concentration of drug, AUC0-168, and minimum concentration of drug on day 1 are 19.2 mcg/mL, 827 mcg • h/mL, and 2.4 mcg/mL, respectively. Rezafungin has a volume of distribution of 67 L and is highly protein bound. It is metabolized by hydroxylation of the terphenyl–pentyl ether side chain to form three hydroxylated metabolite isomers: 2´-, 3´-, and 4´-hydroxypentyl. Rezafungin is also metabolized by O-dealkylation of the pentyl group. Hepatic metabolism has not been observed, and clinically relevant CYP450 drug interactions are not anticipated. Rezafungin is excreted primarily fecally (>70%), chiefly as the parent drug; the fraction excreted renally consists mainly of inactive metabolites. Rezafungin has an elimination clearance of 0.35 L/hour and a terminal half-life of 152 hours. Age, sex, race, weight, and hepatic impairment appear not to have significant effects on rezafungin’s pharmacokinetics.
Adverse Reactions and Drug Interactions9,10: The most common adverse reactions reported for rezafungin (>5%) included hypokalemia, pyrexia, diarrhea, anemia, vomiting, nausea, hypomagnesemia, abdominal pain, constipation, and hypophosphatemia. Rezafungin carries a warning regarding infusion-related reactions (flushing, sensation of warmth, urticaria, nausea, or chest tightness); if these occur, the infusion should be slowed or paused. Because of photosensitivity, patients must avoid exposure to the sun and other sources of UV radiation while receiving rezafungin. Liver-test abnormalities were seen in clinical trials; therefore, patients who develop abnormal liver tests should be monitored and the risks and benefits of continuing therapy weighed.
Rezafungin is not a substrate, inhibitor, or inducer of CYP enzymes or drug-transporter systems. Drug-interaction studies revealed no expected significant effects of rezafungin on the pharmacokinetics of other drugs likely to be coadministered (ibrutinib, venetoclax, efavirenz, mycophenolate mofetil).
Dosage and Administration9,10: Rezafungin is supplied as a single-dose vial containing 200 mg as a solid for reconstitution. The recommended dosage is once weekly by IV infusion, with an initial 400-mg loading dose followed by a weekly 200-mg dose thereafter. If a scheduled dose is missed, it should be administered as soon as possible. If the missed dose is administered within 3 days of the assigned day, the next weekly dose may be given on schedule; if the missed dose is administered >3 days after the assigned day, the dosing schedule should be modified to ensure that >4 days pass before the next dose. If the patient is restarted after >2 weeks of missed dosing, treatment should be reinitiated with a 400-mg loading dose. The safety of rezafungin beyond four weekly doses has not been established.
REFERENCES
1. Zavzpret (zavegepant) product information. New York, NY: Pfizer Labs; March 2023.
2. Lipton RP, Croop R, Stock DA, et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: a phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217.
3. Brenzavvy (bexagliflozin) product information. Marlborough, MA: TheracosBio, LLC; January 2023.
4. Halvorsen YDC, Walford GA, Massaro J, et al. A 96-week, multinational, randomized, double-blind, parallel-group, clinical trial evaluating the safety and effectiveness of bexagliflozin as a monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2496-2504.
5. Tzield (teplizumab-mzwv) product information. Red Bank, NJ: Provention Bio, Inc; November 2022.
6. Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613.
7. Leqembi (lecanemab-irmb) product information. Nutley, NJ: Eisai Inc; January 2023.
8. Van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
9. Rezzayo (rezafungin) product information. Lincolnshire, IL: Melinta Therapeutics LLC; March 2023.
10. Thompson GR III, Soriano A, Cornely OA, et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet. 2023;401(10370):49-59.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





