US Pharm. 2022;47(1):34-42.
ABSTRACT: The utility of antibody-drug conjugates (ADCs) in treating malignancies has continued to grow. Currently, 12 ADCs have been FDA approved to treat hematologic or solid malignancies. Although they are similar in structure and concept, each drug is unique in its indication and molecular target. These differences have important ramifications for identifying patients who are candidates to receive therapy as well as for monitoring of potential adverse effects. As the role of ADCs continues to grow, pharmacists—whether hospital, ambulatory, or community—will continue to be involved in the optimization of care for patients receiving these therapies. It is critical that all pharmacists be familiar with ADCs in order to ensure appropriateness of therapy as well as to mitigate or treat toxicity.
In recent years there have been many therapeutic advances in the field of oncology, including the development and delivery technology of monoclonal antibodies. Upon interaction of an antibody with an antigen (or receptor/protein), several different downstream pathways are activated, including but not limited to neutralization, proapoptotic signaling, antibody-dependent cell-mediated cytotoxicity, antibody-dependent phagocytosis, and complement-dependent cytotoxicity.1 Such mechanisms are typically outlined in an individual drug’s prescribing information (PI).
The use of antibodies to deliver chemotherapy to targeted cells is becoming more common via the use of antibody-drug conjugates (ADCs). Structurally, as depicted in FIGURE 1, ADCs contain an antibody (specific to one site), linker, and payload drug.2 When the antibody component of an ADC binds to its target site, the complex is internalized into the target cell and the payload drug (i.e., chemotherapy) is released to exert a cytotoxic effect on the cell.2 There are varying payload drug-to-antibody ratios (DARs), which impact ADC pharmacokinetics and toxicity profiles.3
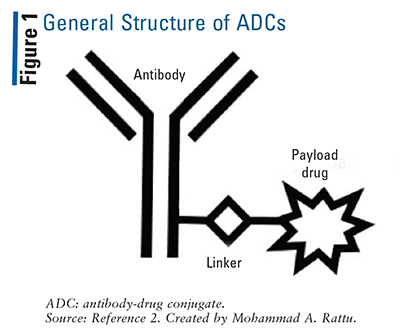
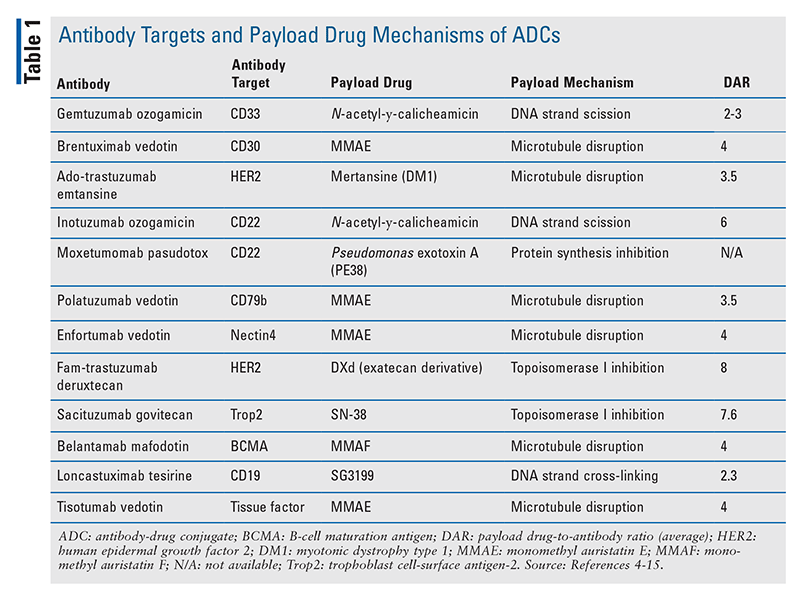
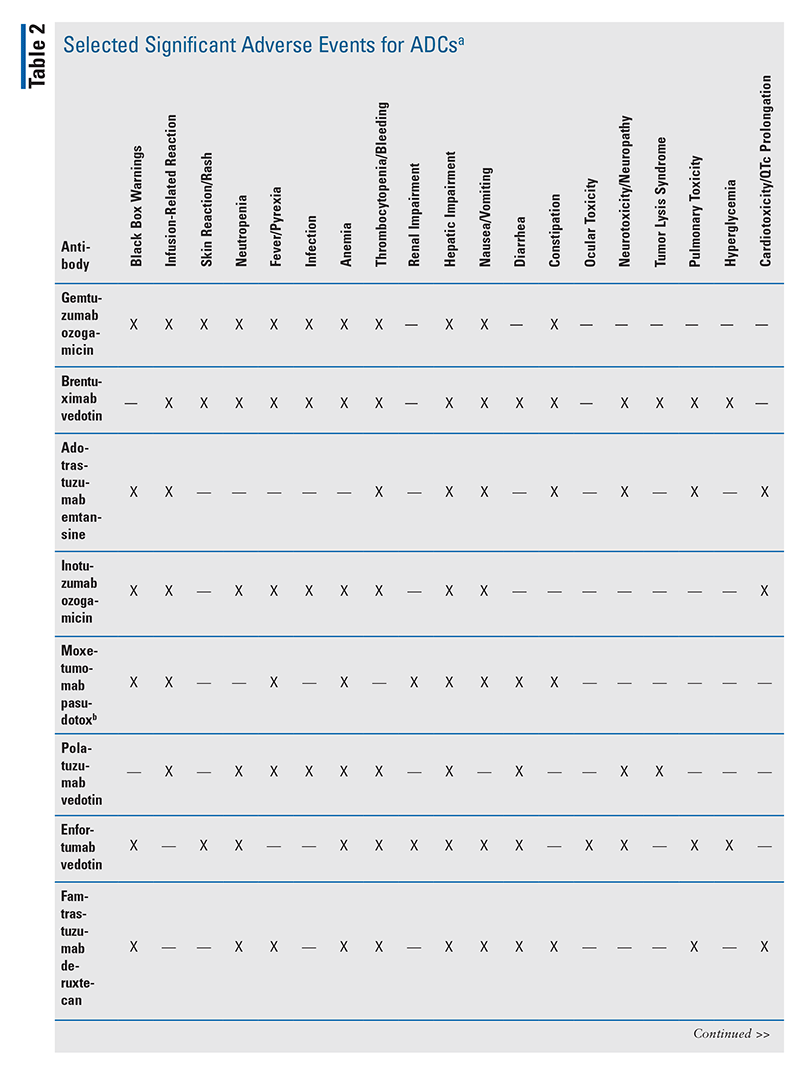
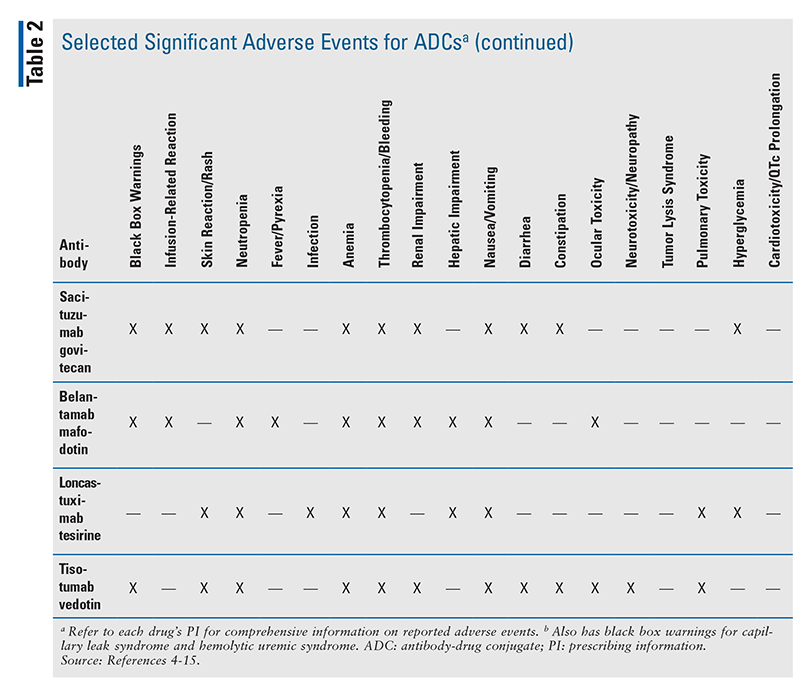
Currently, there are 12 FDA-approved ADCs (see TABLE 1 for information on their antibody targets and payload drug mechanisms).4-15 In addition to understanding each drug’s indication and dosing, pharmacists play a major role in ensuring that appropriate dose adjustments are made (considering drug interactions and laboratory parameters) and supportive-care medications are prescribed to mitigate anticipated adverse effects. TABLE 2 summarizes the toxicity profiles of these 12 ADCs. To enhance the relevance of this comprehensive topic for pharmacists, this article will highlight the pivotal trials that led to FDA approval, black box warnings, and basic treatment strategies for selected adverse events of ADCs. Setting-specific hypothetical scenarios that involve ADCs will also be discussed.
FDA-Approved ADCs
Gemtuzumab ozogamicin (Mylotarg): Gemtuzumab ozogamicin, the first FDA-approved ADC, was withdrawn from the market in 2010 based on negative results from the confirmatory phase III trial S0106.16 However, the drug was reapproved in 2017, with newer data demonstrating positive outcomes with the use of lower doses and different dosing schedules.4 The ALFA-0701, AAML0531, and AML-19 pivotal trials supported its use in newly diagnosed CD33-positive acute myeloid leukemia, and the MyloFrance-1 pivotal trial provided data for the relapsed/refractory setting.4,17-20 Gemtuzumab ozogamicin has a black box warning for hepatotoxicity, including hepatic sinusoidal obstruction syndrome (SOS)/veno-occlusive disease (VOD).4 It is prudent to monitor liver function tests and assess for signs and symptoms of SOS/VOD during therapy. No therapies are proven to mitigate SOS/VOD; if the phenomenon occurs, defibrotide may be needed.21
Brentuximab vedotin (Adcetris): The CD30-targeted ADC brentuximab vedotin received initial FDA approval in 2011, and additional approvals followed in 2017 and 2018.5 Pivotal trials included ECHELON-1 (in previously untreated stage 3 or 4 classical Hodgkin’s lymphoma [cHL]); AETHERA (for consolidation after autologous hematopoietic stem cell transplant [HSCT] in cHL); NCT00848926 (for relapsed cHL); ECHELON-2 (in previously untreated systemic anaplastic large cell lymphoma [ALCL] or other CD30-expressing peripheral T-cell lymphomas [PTCL], including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified); NCT00866047 (in relapsed ALCL); and ALCANZA (in primary cutaneous ALCL and CD30-expressing mycosis fungoides).5,22-27 This drug carries a black box warning for John Cunningham (JC) virus infection, which can lead to progressive multifocal leukoencephalopathy, and there are currently no preventative medications for JC virus.5 Brentuximab vedotin should not be combined with bleomycin, as there may be additive pulmonary toxicity.5 A drug interaction exists between the ADC payload drug monomethyl auristatin E (MMAE) and CYP3A4 inhibitors/inducers.5 Dose adjustments are not mentioned in the PI; therefore, if a strong CYP3A4 inhibitor (e.g., voriconazole) is used concomitantly with brentuximab vedotin, the patient should be monitored more closely for adverse events.5
Ado-trastuzumab emtansine (Kadcyla): Initially FDA-approved in 2013, ado-trastuzumab emtansine was the first ADC used for human epidermal growth factor receptor 2 (HER2)–positive breast cancer.6 Pivotal trials included EMILIA (in HER2-positive metastatic breast cancer after therapy with a taxane and trastuzumab) and KATHERINE (in HER2-positive early breast cancer with residual invasive disease after therapy with neoadjuvant taxane and trastuzumab).6,28,29 The drug carries black box warnings for hepatotoxicity, left ventricular ejection fraction (LVEF) reduction, and embryo-fetal toxicity.6 It is prudent to monitor liver function tests during therapy. LVEF should be checked at baseline, and information on subsequent monitoring and management of signs and symptoms of heart failure is listed in the PI.6 Women of childbearing age should be advised of the risk of fetal harm and the need for effective contraception.6 The payload drug mertansine (DM1), is metabolized by CYP3A4; therefore, treatment with ado-trastuzumab emtansine may need to be delayed until strong CYP3A4 inhibitors (e.g., voriconazole) are cleared from circulation.6 Otherwise, patients should be monitored closely for adverse effects if concomitant use of ado-trastuzumab emtansine and a strong CYP3A4 inhibitor is warranted.6
Inotuzumab ozogamicin (Besponsa): Inotuzumab ozogamicin became the first CD22-targeted ADC approved for B-cell precursor acute lymphoblastic leukemia (ALL) based on the pivotal trial INO-VATE ALL.7,30 There are black box warnings for hepatotoxicity (similar to gemtuzumab ozogamicin) and higher rates of post-HSCT nonrelapse mortality.7 It is prudent to monitor liver function tests during therapy. There are no proven therapies to mitigate SOS/VOD from inotuzumab ozogamicin; if this phenomenon occurs, defibrotide may be needed.31 Additionally, inotuzumab ozogamicin can prolong the QTc interval.7 Typical mitigation strategies include minimizing concomitant drugs that prolong the QTc interval (e.g., ondansetron), optimizing electrolytes (e.g., magnesium, potassium), and avoiding bradycardia.7,32
Moxetumomab pasudotox (Lumoxiti): The FDA approved moxetumomab pasudotox in 2018 for patients with relapsed/refractory hairy cell leukemia who previously received two or more prior systemic therapies, including a purine nucleoside analogue (e.g., cladribine, pentostatin), based on the pivotal trial NCT01829711.8,33 Structurally, moxetumomab pasudotox contains scFv (single chain immunoglobulin G antibody fragment with heavy and light chain domains) with affinity for CD22, which is covalently bonded to Pseudomonas exotoxin A.8,34-36 Given its antibody fragment and payload drug structure—as opposed to a whole antibody and linker—some references list moxetumomab pasudotox as an immunotoxin rather than an ADC.37 This drug has black box warnings for capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS).8 Body weight, blood pressure, albumin, and signs or symptoms of third-spacing (e.g., peripheral or pulmonary edema) should be monitored for CLS.8 Hemoglobin, platelets, and serum creatinine should be monitored for HUS.8 If HUS is suspected, the hemolysis workup should include a blood smear to identify schistocytes and labs for lactate dehydrogenase and indirect bilirubin.8 Detailed grading and management of both CLS and HUS are discussed in the PI.8
Polatuzumab vedotin (Polivy): In 2019, the FDA approved the CD79b-targeted ADC polatuzumab vedotin, in combination with bendamustine and rituximab, for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more prior lines of therapy based on the pivotal trial GO29365.9,38 Similar to brentuximab vedotin, there exists a drug interaction between the MMAE component of polatuzumab vedotin and strong CYP3A4 inhibitors/inducers, and close monitoring for adverse effects is warranted.9
Enfortumab vedotin (Padcev): The Nectin4-directed ADC enfortumab vedotin was FDA approved in 2019 for adult patients with metastatic urothelial cancer who previously received a programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitor and platinum-containing chemotherapy (based on the pivotal trial EV-301) as well as in patients who are ineligible for cisplatin-containing chemotherapy and previously received one or more prior lines of therapy (based on the pivotal trial EV-201).10,39,40 Enfortumab vedotin carries a black box warning for cutaneous reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis.10 If such skin reactions are suspected, the drug should be held and a dermatologist should be consulted to confirm the diagnosis.10 Although not listed as a black box warning, ocular toxicity (e.g., dry eyes, blurred vision) can occur with enfortumab vedotin, and prophylactic artificial-tears eyedrops and an ophthalmologic evaluation are warranted.10 Similar to brentuximab vedotin and polatuzumab vedotin, there exists a drug interaction between the MMAE component of enfortumab vedotin and strong CYP3A4 inhibitors/inducers, and close monitoring for adverse effects is warranted.10
Fam-trastuzumab deruxtecan (Enhertu): In 2019, fam-trastuzumab deruxtecan was the second HER2-directed ADC approved for breast cancer, and in 2021 it became the first ADC approved for gastric cancer.11 Pivotal trials include DESTINY-Breast01 (in patients with unresectable or metastatic breast cancer who received two or more prior HER2-directed regimens) and DESTINY-Gastric01 (in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma who received a prior trastuzumab-containing regimen).11,41,42 Fam-trastuzumab deruxtecan has black box warnings for pulmonary complications (including interstitial lung disease and pneumonitis) and for embryo-fetal toxicity.11 Patients should be monitored for signs or symptoms of respiratory toxicities, including cough, dyspnea, and/or fever.11 Women of childbearing age should be advised of the risk of fetal harm and the need for effective contraception.11 Although LVEF reduction is not listed as a black box warning (as with ado-trastuzumab emtansine), it remains prudent to check LVEF at baseline. Information on subsequent monitoring and management of signs and symptoms of heart failure is given in the PI.11 Additionally, strong CYP3A4 or organic anion transporting polypeptide inhibitors (e.g., ritonavir) can increase levels of the DXd component in the ADC, although this interaction is stated as not clinically meaningful.11
Sacituzumab govitecan (Trodelvy): Sacituzumab govitecan targets and binds to trophoblast cell-surface antigen-2 and delivers the payload drug SN38—which is the active metabolite of irinotecan—to target cells. The FDA approved sacituzumab govitecan for triple-negative breast cancer in 2020 and urothelial cancer in 2021.12 Pivotal trials included ASCENT/IMMU-132-01 (in patients with locally advanced or metastatic triple negative breast cancer who received two or more prior systemic therapies) and TROPHY/IMMU-132-06 (in patients with locally advanced or metastatic urothelial cancer who previously received a PD-1/PD-L1 inhibitor and platinum-containing chemotherapy).12,43,44 The active chemotherapeutic agent is SN38, so UDP-glucuronosyltransferase (UGT)1A1 polymorphism issues and drug interactions (inhibition/induction of UGT1A1) are mentioned in the PI.12 Similar to irinotecan, the ADC sacituzumab govitecan carries black box warnings for neutropenia and diarrhea.12 CBC should be closely monitored, and therapy should be held for an absolute neutrophil count (ANC) <1,500/mm3 or for neutropenic fever.12 Granulocyte colony-stimulating factor (e.g., filgrastim) prophylaxis should be considered for subsequent doses in order to prevent therapy delays secondary to low ANC, and anti-infective treatment with broad-spectrum antimicrobials should be promptly administered in the event of neutropenic fever.12 Severe diarrhea may occur. Acute or early episodes of diarrhea may require the use of atropine if not otherwise contraindicated.12 For late-onset diarrhea, consider both infectious and noninfectious etiologies. Infectious diarrhea should be treated appropriately upon identification of a causative organism; otherwise, if diarrhea is noninfectious, loperamide may be initiated to ameliorate symptoms.12 Detailed dose adjustments for neutropenia and diarrhea are listed in the PI.12
Belantamab mafodotin (Blenrep): The FDA approved belantamab mafodotin for relapsed/refractory multiple myeloma (MM) in patients who previously received four or more prior lines of therapy, including anti-CD38 monoclonal antibody, proteasome inhibitor, and immunomodulatory agent, based on the pivotal trial DREAMM-2.13,45 The antibody targets B-cell maturation antigen and is conjugated to monomethyl auristatin F. The drug has a black box warning for ocular toxicity (e.g., corneal epithelium changes, vision changes, dry eyes); therefore, an ophthalmic examination is required at baseline, prior to each dose, and if any worsening ocular symptoms present during therapy.13 Patients should use preservative-free lubricant eyedrops and avoid contact lenses unless otherwise directed by an ophthalmologist.13 Belantamab mafodotin is the only ADC with a Risk Evaluation and Mitigation Strategy (REMS) safety program to manage the risk of ocular toxicity.13 Similar to other REMS programs, patients must be enrolled, and prescribers and healthcare settings must be certified prior to drug administration.13
Loncastuximab tesirine (Zynlonta): The CD19-targeted ADC loncastuximab tesirine was FDA approved in early 2021 for patients with relapsed/refractory DLBCL who previously received two or more prior systemic therapies based on the pivotal trial LOTIS-2.14,46 The payload drug, SG3199, is a p-glycoprotein (P-gp) substrate, but currently the PI contains no data on dose adjustments when the ADC is combined with P-gp inhibitors/inducers.14 Closer monitoring for adverse events would likely be warranted with this combination.
Tisotumab vedotin (Tivdak): Most recently (September 2021), based on the pivotal trial InnovaTV-204, the FDA approved the tissue factor–directed ADC tisotumab vedotin for patients with recurrent/metastatic cervical cancer with disease progression on or after chemotherapy.15,47 Similar to belantamab mafodotin, tisotumab vedotin has a black box warning for ocular toxicity (e.g., corneal epithelium changes, vision changes, dry eyes), and ophthalmic examinations are required at baseline, prior to each dose, and as clinically indicated.15 The PI contains an extensive review of the required eye care.15 Similar to the other ADCs with MMAE (e.g., brentuximab vedotin, polatuzumab vedotin, enfortumab vedotin), a drug interaction exists between the MMAE component of tisotumab vedotin and strong CYP3A4 inhibitors.15 There are no recommended dose adjustments for this interaction, and patients should be monitored closely for adverse effects.15
Hypothetical Case 1
Outpatient, Community Pharmacy: EG is a 60-year-old male with a history of bladder cancer, diabetes, and hypertension. His primary oncologist recently initiated enfortumab vedotin, and EG has completed 2 weeks of treatment thus far. He presents to the community pharmacy with new prescriptions for artificial-tears eyedrops and a higher dose of metformin. The prescriptions are covered by EG’s insurance, and he declines counseling when he picks up his medications. About 2 weeks later, EG returns to the pharmacy with prescriptions for prednisolone acetate 1% eyedrops and hydrocortisone 2% ointment. He complains about making frequent trips to the pharmacy. The pharmacist asks EG about the situation. EG states that his new chemotherapy has been making his vision worse and his diabetes is now uncontrolled despite minimal carbohydrate intake, and he just developed a small rash on his chest (although he has no history of allergy). The pharmacist validates EG’s concern that these issues might be related to enfortumab vedotin and recommends that EG discuss these adverse effects in detail with his primary oncologist. The pharmacist counsels EG on the proper administration of eyedrops and topical corticosteroids and ascertains that EG has a functional glucose monitor and adequate unexpired test strips at home.
Discussion: This case highlights the ocular, metabolic, and dermatologic toxicities of enfortumab vedotin. In clinical trials, the median time to onset of ocular symptoms was 1.6 months (range: 0-19.1 months), with 40% of patients developing some type of ocular disorder.10 In addition, patients with a hemoglobin A1C >8% were excluded and 14% of patients developed hyperglycemia.10 For blood glucose levels >250 mg/dL, the PI suggests holding enfortumab vedotin until improvement is seen.10 EG could have received insulin prior to his scheduled ADC dose, along with titration of his maintenance metformin dose. In clinical trials, 55% of patients developed skin reactions.10 EG’s rash did not fit the criteria for a grade >3 skin reaction, and management included topical corticosteroids.10,48
Hypothetical Case 2
Outpatient, Ambulatory Clinic: VZ is a 65-year-old female with a history of MM, lower-extremity deep vein thrombosis, chronic kidney disease, and peripheral neuropathy. Her MM is progressing by persistent free kappa light chain elevation despite numerous previous treatment combinations including daratumumab, bortezomib, lenalidomide, dexamethasone, carfilzomib, and pomalidomide. VZ does not want to be admitted to the hospital at this time, whether for infusional chemotherapy (e.g., DCEP [dexamethasone, cyclophosphamide, etoposide, cisplatin]) or HSCT (e.g., melphalan). VZ also has had significant difficulties with nausea and vomiting during prior chemotherapy regimens, and she is not in favor of selinexor for that reason; she is willing to try belantamab mafodotin. The outpatient clinical pharmacist works with the primary oncologist to ensure that REMS enrollment is completed and that VZ’s laboratory parameters are acceptable for proceeding with treatment. VZ is preemptively started on preservative-free artificial-tears eyedrops. During the first infusion of belantamab mafodotin, VZ experiences anxiety and shortness of breath. The infusion is interrupted, resumed at a lower rate, and completed without further problems. One month later, VZ is informed that her platelet count is <50,000/mcL and that the ADC dose will be reduced for the second cycle. The following month, VZ’s primary oncologist notes that there has been only a partial response to belantamab mafodotin and strongly recommends hospital admission for DCEP.
Discussion: This case highlights the utility of belantamab mafodotin in the relapsed/refractory MM setting as well as mitigation strategies for ocular toxicity, infusion-related reactions, and thrombocytopenia. In clinical trials, ocular adverse reactions occurred in 77% of patients; therefore, preservative-free artificial-tears eyedrops should be initiated with the first ADC dose (and should be continued as long as indicated by an ophthalmologist).13 VZ did not manifest any overt ocular issues. In clinical trials, infusion-related reactions occurred in 18% of patients; therefore, if a grade 2 or 3 reaction occurs, the infusion should be paused, supportive care provided, and the infusion resumed at a lower rate.13 Routine premedications (e.g., acetaminophen, diphenhydramine) are not explicitly recommended in the PI.13 VZ experienced an infusion-related reaction but was able to complete the first infusion and the subsequent dose without any further problems. In clinical trials, thrombocytopenia occurred in 69% of patients, which—depending on the severity—may warrant dose reduction, interruption, or discontinuation.13 VZ was given a lower ADC dose to mitigate thrombocytopenia, but additional interventions could have included a platelet transfusion and/or cessation of concomitant drugs that can affect platelets (e.g., aspirin, anticoagulants).
Hypothetical Case 3
Inpatient, Hospital: PR is a 35-year-old male with a history of relapsed/refractory B-cell precursor ALL, depression, and type 1 diabetes mellitus (T1DM). His primary oncologist recommends inpatient initiation of inotuzumab ozogamicin. On day 1, PR is given premedications (including acetaminophen, diphenhydramine, and hydrocortisone) prior to inotuzumab ozogamicin. He experiences a mild hyperglycemic event, which is treated with a titrated regimen of insulin lispro and glargine. Prior to receiving inotuzumab ozogamicin on day 8, PR has a bradycardic event with a heart rate <50, but his blood pressure is normal. This prompts the team to check an ECG, which reveals sinus bradycardia and a QTc interval of 480 ms. The pharmacist on rounds informs the team of the increased risk of further QTc prolongation with concomitant use of the ADC and escitalopram. The team consults the psychiatrist about potentially reducing the escitalopram dose (or switching to an alternative), supplementing magnesium (to a goal >2 mg/dL) and potassium (to a goal >4 mEq/L), and proceeding with the day-8 dose of inotuzumab ozogamicin. By day 15, the team would have discharged PR, but he developed neutropenic fever on day 11 and has remained on cefepime.
Discussion: This case highlights a situation in which a patient may be admitted to initiate an ADC, particularly for potential toxicity. PR did not have an infusion-related reaction (reported in 2% of patients in clinical trials), but he experienced a hyperglycemic event from hydrocortisone.7 Considering PR’s history of T1DM, the most appropriate intervention for this situation remains insulin. In clinical trials, QTc prolongation was documented in 3% of patients.7 PR was on escitalopram (for depression), a selective serotonin reuptake inhibitor known to increase the risk of QTc prolongation. In clinical trials, there was a significant incidence of neutropenia (49%) and neutropenic fever (26%).7 The most appropriate first-line parenteral antibiotic for neutropenic fever should cover Pseudomonas aeruginosa (e.g., cefepime, piperacillin-tazobactam).
Conclusion
The current FDA-approved ADCs have been studied in a variety of hematologic and solid malignancies. Despite the similarity in concept, the indications, specific molecular targets, and chemotherapy/therapeutic agents vary between products. The number of available therapies is anticipated to continue growing. It is critical that pharmacists evaluate patients for appropriateness of therapy prior to initiation as well as recommend prophylactic medications to mitigate toxicity. Pharmacists will continue to play an evolving role in the identification and safe administration of these products for their patients.
REFERENCES
1. Cuesta-Mateos C, Alcaraz-Serna A, Somovilla-Crespo B, Muñoz-Calleja C. Monoclonal antibody therapies for hematological malignancies: not just lineage-specific targets. Front Immunol. 2018;8:1936.
2. Khongorzul P, Ling CJ, Khan FU, et al. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3-19.
3. Sun X, Ponte JF, Yoder NC, et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjug Chem. 2017;28(5):1371-1381.
4. Mylotarg (gemtuzumab ozogamicin) package insert. Philadelphia, PA: Wyeth Pharmaceuticals LLC; August 2021.
5. Adcetris (brentuximab vedotin) package insert. Bothell, WA: Seagen Inc; October 2019.
6. Kadcyla (ado-trastuzumab emtansine) package insert. South San Francisco, CA: Genentech, Inc; September 2020.
7. Besponsa (inotuzumab ozogamicin) package insert. Philadelphia, PA: Wyeth Pharmaceuticals LLC; March 2018.
8. Lumoxiti (moxetumomab pasudotox-tdfk) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP, Inc; October 2021.
9. Polivy (polatuzumab vedotin-piiq) package insert. South San Francisco, CA: Genentech, Inc; September 2020.
10. Padcev (enfortumab vedotin-ejfv) package insert. Bothell, WA: Seagen Inc; November 2021.
11. Enhertu (fam-trastuzumab deruxtecan-nxki) package insert. Basking Ridge, NJ: Daiichi Sankyo Inc; January 2021.
12. Trodelvy (sacituzumab govitecan-hziy) package insert. Morris Plains, NJ: Immunomedics Inc; April 2021.
13. Blenrep (belantamab mafodotin-blmf) package insert. Research Triangle Park, NC: GlaxoSmithKline; August 2020.
14. Zynlonta (loncastuximab tesirine-lpyl) package insert. Murray Hill, NJ: ADC Therapeutics America; September 2021.
15. Tivdak (tisotumab vedotin-tftv) package insert. Bothell, WA: Seagen Inc; September 2021.
16. Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and post consolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854-4860.
17. Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508-1516.
18. Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021-3032.
19. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
20. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
21. Gemtuzumab ozogamicin. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
22. Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378(4):331-344.
23. Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853-1862.
24. Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562-1566.
25. Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240.
26. Pro B, Advani R, Brice P, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709-2717.
27. Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555-566.
28. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783-1791.
29. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628.
30. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753.
31. Inotuzumab ozogamicin. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
32. Cohagan B, Brandis D. Torsade de pointes. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021.
33. Kreitman RJ, Dearden C, Zinzani PL, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32(8):1768-1777.
34. Vallera DA, Kreitman RJ. Immunotoxins targeting B cell malignancy-progress and problems with immunogenicity. Biomedicines. 2018;7(1):1.
35. Nelson AL. Antibody fragments: hope and hype. MAbs. 2010;2(1):77-83.
36. Weldon JE, Pastan I. A guide to taming a toxin—recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278(23):4683-4700.
37. Moxetumomab pasudotox. Lexicomp Online [online database]. Hudson, OH: Lexi-Comp, Inc; 2017. https://online.lexi.com. Accessed October 31, 2021.
38. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165.
39. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125-1135.
40. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592-2600.
41. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621.
42. Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419-2430.
43. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541.
44. Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474-2485.
45. Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207-221.
46. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790-800.
47. Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609-619.
48. National Cancer Institute Division of Cancer Treatment & Diagnosis. Common terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed October 31, 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






