US Pharm. 2023;48(10):34-38.
ABSTRACT: Immunization is one of the most cost-effective measures for the prevention of infectious disease. During the COVID-19 pandemic, Public Readiness and Emergency Preparedness Act amendments expanded the authority of pharmacists, pharmacy technicians, and interns to administer vaccines, bringing attention to the profession’s role in this area. Laws regarding pharmacist authority are state-determined, so pharmacists must keep abreast of the current regulations in their state. Despite the accessibility of community pharmacies and widespread vaccine availability, vaccination rates continue to be suboptimal. Pharmacists are well positioned to identify patients who would benefit from vaccinations; they can also provide education, screening, and documentation, and they can be instrumental in allaying concerns by counseling patients and caregivers in making the best-informed choice.
Immunization is one of the most cost-effective measures for preventing infectious disease. The COVID-19 pandemic provided evidence of the vital role of pharmacists in delivering this preventative health measure. During the pandemic, amendments to the Public Readiness and Emergency Preparedness (PREP) Act expanded the authority of pharmacists, pharmacy technicians, and interns to administer vaccines, bringing attention to the profession’s role in this area.1
Most of the U.S. population resides within 5 miles of a pharmacy, enabling access to vaccines and other healthcare services that pharmacists provide.2 Despite the widespread availability of vaccines, vaccination rates remain suboptimal owing to the lack of patient education, especially in underserved communities, along with inconsistencies in state laws governing administration by pharmacists. Pharmacists have proven to be qualified providers, advocates, and educators and have a major role in promoting the importance of immunizations. Government policies that allow pharmacists to administer all Advisory Committee on Immunization Practices (ACIP)–recommended, FDA-approved vaccines are essential. These policies must include vaccine accessibility for all patients, along with appropriate reimbursement, allowing pharmacists to continue to play a key role in this initiative.3
Pharmacists’ involvement in immunization began with distribution of the smallpox vaccine in the 1800s, and the first formalized training for vaccine administration by pharmacists was in 1994.4,5 In November 1996, the American Pharmacists Association initiated its vaccine certificate program for pharmacists, which continues to be used by numerous colleges of pharmacy and other organizations.4,5 This program educates participants on topics such as basic immunology, vaccine-preventable diseases, implementation of vaccine delivery into practice, regulatory and legal issues, strategies for improving immunization rates, and administration techniques.6
Pharmacists’ immunization practices are also expanding to include the supervision of pharmacy technicians in administering vaccines. Prior to the 2020 PREP Act, which authorized trained pharmacy technicians to administer some immunizations, several states had enacted legislation to allow trained and qualified pharmacy technicians to administer vaccinations.7 At least 11 training programs that focus on vaccine-administration techniques for pharmacy technicians are currently available.7 Although pharmacists are well positioned to administer vaccinations, they are underutilized; some of the barriers include variances in state laws, collaborative protocol requirements, pharmacy staffing limitations, and health plan payment restrictions.3
Vaccines as Preventative Care
Vaccines are one of the most cost-effective measures for protection against certain diseases, and it is estimated that they save 2.5 million lives annually.3 The ACIP recommends vaccination against a variety of infectious diseases for U.S. adults and children. Immunization schedules should always be checked for the most up-to-date recommendations. After being approved by the ACIP, immunization schedules are published in the CDC’s Morbidity and Mortality Weekly Report and also appear on the CDC’s website.8 In addition to routine vaccinations, pharmacists can assist patients who are traveling abroad with vaccines recommended for their destination. The CDC’s Travelers’ Health website identifies the recommended vaccines for different countries.9
Pharmacists’ Role in Immunization Practices
Pharmacists are in a unique position to identify patients who would benefit from vaccinations. They can also provide education, screening, and documentation and can be instrumental in allaying concerns by counseling patients and caregivers in making the best-informed choice. Pharmacists are often the most accessible health professional, especially in rural areas. Community pharmacists are highly visible and available to the public, and their scope of practice in the U.S. continues to grow.10
Community pharmacists are especially well situated to increase vaccine availability and expand their utility. The COVID-19 pandemic significantly expanded the responsibilities of pharmacists in the U.S., and surveys have supported pharmacists’ interest in a larger role in vaccine administration.11 During the 2009 H1N1 pandemic, community pharmacies were underused as vaccination sites; since then, however, approximately one-third of adults have been obtaining their influenza vaccinations at pharmacies.12 Pharmacists’ involvement as advocates and vaccine administrators in the U.S. has demonstrated positive effects on immunization acceptance, especially for influenza.13 The COVID-19 pandemic highlighted the pharmacy’s impact on patient vaccination, and a recent JAMA Health Forum article noted that more than 300 million doses of COVID-19 vaccine were provided by community pharmacies between early 2021 and May 2023.14,15
Opportunities for involvement in immunization practice exist outside the community pharmacy and beyond vaccine administration. In healthcare settings other than community pharmacies, long-term-care consultant pharmacists have contributed to the increased number of pneumococcal immunizations in these facilities.16
Although pharmacists have had a positive impact on vaccine access, their participation in state and federal vaccination programs, including the Vaccines for Children program, continues to lag. Barriers to pharmacist participation include reimbursement, regulatory issues, and role-based restrictions. Changes are essential in these areas to achieve improved rates of vaccination in the pediatric population.17
Pharmacists as Vaccine Advocates
Many people, including parents of young children, need education about the healthcare that community pharmacists can provide, including vaccinations.18 Misinformation from various sources has influenced vaccine-administration rates nationwide. The educational services pharmacists provide can enhance public awareness and willingness to obtain vaccinations.19
PREP Act’s Impact on Pharmacy Vaccination Services
The PREP Act measures undertaken during the COVID-19 pandemic furthered pharmacies’ impact on vaccination services. The PREP Act gave pharmacists, pharmacy technicians, and pharmacy students the authority to administer COVID-19 and influenza immunizations to adults and children aged 3 years and older. The U.S. Department of Health and Human Services recently announced that this authorization will continue through December 2024.1
Variations in State Vaccine Regulations
Although all U.S. states permit pharmacists to administer vaccines, state laws differ on the details of this authorization. PREP Act authorizations have precedence over state regulations during times of public emergency, which included the COVID-19 pandemic. Several vaccine-authorization factors vary from state to state, including which vaccines can be administered, age limitations, and prescription requirements for individual vaccines. The National Alliance of State Pharmacy Associations provides periodic updates on states’ regulations concerning pharmacist immunization authority.20 TABLE 1 lists age-related regulations for pharmacist-administered influenza and COVID-19 vaccines in each state.20
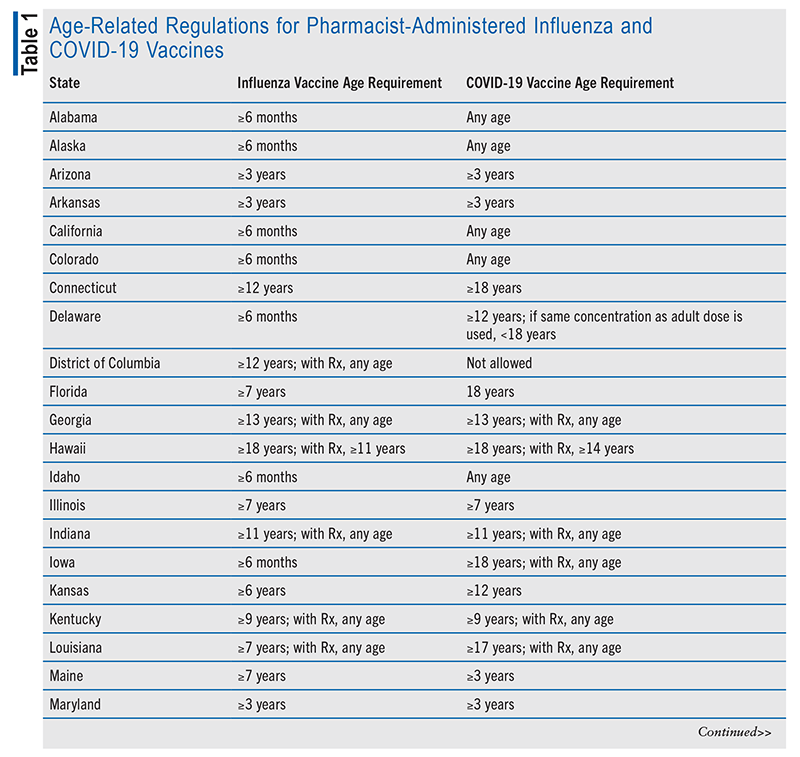
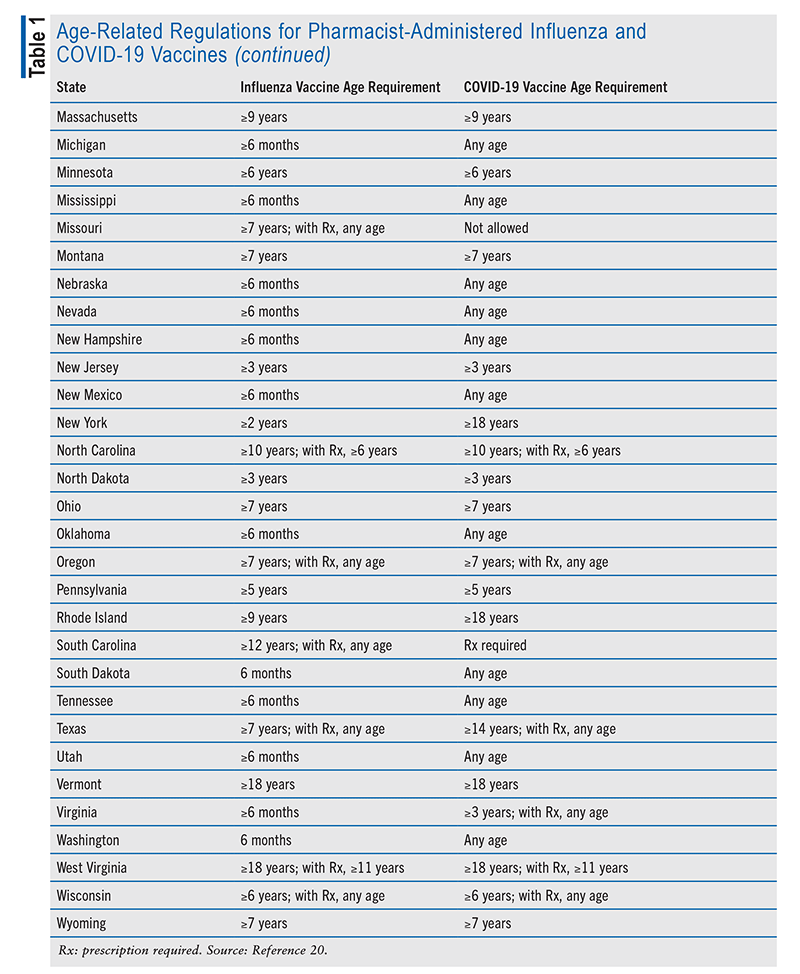
Prescription requirements and administration authority for routine vaccinations in adults (defined here as vaccines listed on the CDC adult immunization schedule, other than influenza and COVID-19) also differ among states. Seventeen states allow pharmacists to prescribe vaccines, and 32 states allow administration via a prescriber-approved protocol or non–patient-specific order.20 Some states have specific requirements for certain vaccines. Examples include Missouri’s requirement for a prescription for Haemophilus influenzae, human papillomavirus, MMR (measles, mumps, and rubella), and varicella vaccines and North Carolina’s requirement for a prescription for H influenzae, MMR, and varicella vaccines. In the District of Columbia, pharmacists are not allowed to administer MMR and varicella vaccines.
Prescription requirements and administration authority for routine childhood vaccinations differ even more among states. Examples include 18 states not authorizing pharmacists to administer routine vaccines to patients younger than age 7 years and varying age restrictions in other states.20
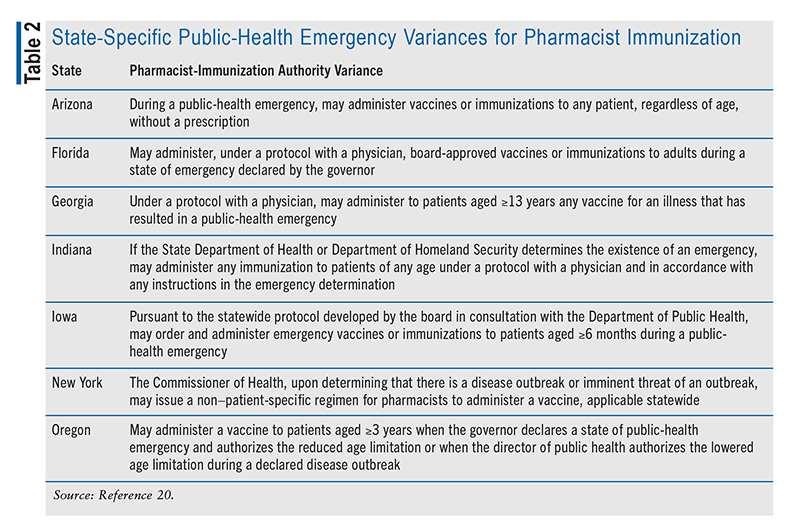
Pharmacists need to keep abreast of the state regulations regarding vaccine practices for each state in which they practice.20 State-specific rules also apply to pharmacist involvement regarding the mpox vaccines made available for cases reported in 2022; 37 states permit pharmacists to prescribe or administer these vaccines under a prescriber-approved protocol, and eight states allow mpox vaccination by pharmacists, albeit with additional requirements. Some states have individual variances for administration of the vaccine by pharmacists during a public-health emergency (TABLE 2).20
Pharmacists’ Ongoing Education
After obtaining immunization certification, pharmacists should be aware of their state’s requirements for immunization-related continuing education. Some states now require completion of immunization-specific credits. The importance of proper education and certification completion by providers who administer vaccinations is crucial to ensuring safety and the prevention of vaccination-related errors.21 Pharmacists are in an excellent position to identify potential vaccine and drug interactions. The COVID-19 pandemic has further highlighted this strength, as knowledge of these potential interactions is essential for making appropriate drug-use decisions.22
Immunization-Information System Use
Pharmacists’ contribution to immunization records grows in importance as their involvement increases. Only 60% of U.S. adults have this data recorded in an immunization-information system, suggesting that there is room for improvement.23 Pharmacists should review immunization-information systems to determine whether patients have vaccine documentation. Currently, a federal immunization-information system does not exist. Therefore, a barrier for pharmacists is a lack of access to state-specific information systems. In 2022, the American Pharmacists Association amended its Proactive Immunization Assessment and Immunization Information Systems policy to include language supporting national integration of these systems.24
Conclusion
The COVID-19 pandemic has highlighted the impact that pharmacists and pharmacy staff have on immunizing the public. PREP Act allowances have expanded the role pharmacy teams play in preventative care and have increased public awareness of pharmacies’ vaccine availability. Because of differences in state regulations, pharmacists should always consult their state Board of Pharmacy or Department of Health to ensure that they are familiar with jurisdictional regulations. Aside from administration, pharmacists also serve a vital purpose in educating the public on vaccines, particularly considering the accessibility of pharmacies in many rural U.S. communities where the availability of other healthcare providers may be limited. Misinformation and distrust of the healthcare system have influenced immunization rates. As front-line healthcare providers, pharmacists can significantly influence vaccination rates through educational incentives, vaccine availability, and documentation for their patients and communities.
REFERENCES
1. U.S. Department of Health and Human Services. Fact sheet: HHS announces intent to amend the declaration under the PREP Act for medical countermeasures against COVID-19. www.hhs.gov/about/news/2023/04/14/factsheet-hhs-announces-amend-declaration-prep-act-medical-countermeasures-against-covid19.html. Accessed July 28, 2023.
2. Strand MA, Bratberg J, Eukel H, et al. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:e69.
3. Poudel A, Lau ETL, Deldot M, et al. Pharmacist role in vaccination: evidence and challenges. Vaccine. 2019;37(40):5939-5945.
4. Richardson WM, Wertheimer AI. A review of the pharmacist as vaccinator. Innov Pharm. 2019;10(3):10.24926/iip.v10i3.940.
5. Hogue MD, Grabenstein JD, Foster SL, Rothholz MC. Pharmacist involvement with immunizations: a decade of professional advancement. J Am Pharm Assoc (2003). 2006;46(2):168-179.
6. American Pharmacists Association. Pharmacy-based immunization delivery. www.pharmacist.com/Education/Certificate-Training-Programs/Immunization. Accessed July 31, 2023.
7. Adams AJ, Bright D, Adams J. Pharmacy technician-administered immunizations: a five-year review. J Am Pharm Assoc (2003). 2022;62(2):419-423.
8. CDC. Immunization schedules. www.cdc.gov/vaccines/schedules/index.html. Accessed July 31, 2023.
9. CDC. Travelers’ health. wwwnc.cdc.gov/travel. Accessed July 31, 2023.
10. Hess K, Bach A, Won K, Seed SM. Community pharmacists roles during the COVID-19 pandemic. J Pharm Pract. 2022;35(3):469-476.
11. Carpenter DM, Hastings T, Westrick S, et al. Rural community pharmacists’ ability and interest in administering COVID-19 vaccines in the Southern United States. J Am Pharm Assoc (2003). 2022;62(4):1379-1383.
12. Gessler CA, Richardson RM, Hall DL, Coley KC. Operationalizing pandemic vaccinations at a regional supermarket chain pharmacy. Disaster Med Public Health Prep. 2022;16(5):2070-2075.
13. Le LM, Veettil SK, Donaldson D, et al. The impact of pharmacist involvement on immunization uptake and other outcomes: an updated systematic review and meta-analysis. J Am Pharm Assoc (2003). 2022;62(5):1499-1513.e16.
14. Grabenstein JD. Essential services: quantifying the contributions of America’s pharmacists in COVID-19 clinical interventions. J Am Pharm Assoc (2003). 2022;62(6):1929-1945.e1.
15. Layson-Wolf C, Sharfstein JM. US community pharmacies and public health—building on the COVID-19 response. JAMA Health Forum. 2023;4(6):e232583.
16. Dauz A, O’Neil CK, Stewart-Lynch A. Assessing the impact of the consultant pharmacist on pneumococcal vaccine administration in a long-term care facility. Sr Care Pharm. 2022;37(11):565-570.
17. Alden J, Crane K, Robinson R, et al. Expansion of community pharmacies’ role in public vaccine delivery to children: opportunities and need. J Am Pharm Assoc (2003). 2022;62(5):1514-1517.
18. Varisco TJ, Downs CG, Sansgiry SS, et al. Parents’ intention to have their child vaccinated at a community pharmacy: a national cross-sectional survey. J Am Pharm Assoc (2003). 2023;63(2):511-517.e8.
19. Bayraktar-Ekincioglu A, Kara E, Bahap M, et al. Does information by pharmacists convince the public to get vaccinated for pneumococcal disease and herpes zoster? Ir J Med Sci. 2022;191(5):2193-2200.
20. National Alliance of State Pharmacy Associations. Pharmacist administered vaccines. https://naspa.us/wp-content/uploads/2021/01/Pharmacist-Immunization-Authority-April-2023.pdf. Accessed July 31, 2023.
21. Poiraud C, Réthoré L, Bourdon O, et al. Understanding and preventing vaccination errors. Infect Dis Now. 2023;53(2):104641.
22. Badary OA. Implications of potential clinically relevant interactions between COVID-19 vaccines and concomitant medications. Rev Med Virol. 2023;33(3):e2417.
23. Hastings TJ, Ha D, Fox BI, et al. Increasing use of immunization information systems for routine vaccinations in independent community pharmacies: a randomized controlled trial. J Am Pharm Assoc (2003). 2022;62(4):1270-1279.e2.
24. American Pharmacists Association. APhA policy manual. https://aphanet.pharmacist.com/policy-manual. Accessed July 31, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






