US Pharm. 2023;48(7):14.
Respiratory syncytial virus (RSV) is highly contagious and affects the lungs and respiratory passages. It usually causes mild, coldlike symptoms that resolve within 1 to 2 weeks but can also cause serious illness in infants and older adults. RSV is the most common cause of bronchiolitis and pneumonia in infants aged <1 year and a leading cause of lower respiratory tract disease (LRTD) among older adults in the United States.
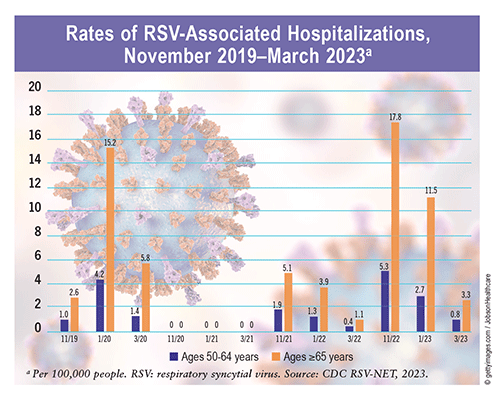
RSV Patterns: RSV circulation is seasonal, typically starting in the fall and peaking during the winter. According to data from 10 U.S. Department of Health and Human Services regions countrywide (excluding Florida and Hawaii), sustained increases in RSV-positive (RSV+) tests were reported from mid-September to mid-November, peak RSV+ tests were reported from late December to mid-February, and sustained decreases in RSV+ tests occurred from mid-April to mid-May annually from 2014 to 2017. These patterns remained consistent in areas with similar climates until the COVID-19 pandemic in early 2020, when the seasonal onset of RSV began to rise during the spring and cases peaked in July. In 2022, RSV activity began in September and peaked in mid-November.
RSV in Older Adults: CDC surveillance data estimate that RSV leads to approximately 60,000 to 160,000 hospitalizations and 6,000 to 10,000 deaths annually in U.S. adults aged >65 years. During the 2022 RSV season, about six of every 100,000 older adults were hospitalized for RSV and related complications—a rate 10 times higher than that for years prior to 2020. A study assessing the incidence of RSV+ acute respiratory infection (ARI) among adults aged >50 years found a substantial RSV+ ARI incidence rate between 2019 and 2020 (48.6/1,000 person-years), a reduction between 2020 and 2021 (0/1,000 person-years), and a reemergence in mid-2021 (10.2/1,000 person-years). RSV+ ARI was also associated with significant long-term decreases in quality of life at 12 to 13 months post infection independent of age range, sex, race/ethnicity, socioeconomic status, and high-risk comorbidities.
RSV Prevention: In May 2023, the FDA approved two protein-based vaccines for RSV: Arexvy (GlaxoSmithKline) and Abrysvo (Pfizer). Both are indicated for the prevention of LRTD caused by RSV in individuals aged >60 years. This is an important public-health achievement benefiting older adults who are at high risk for severe RSV-related illness because of immunodeficiency and/or underlying health conditions such as chronic obstructive pulmonary disease, asthma, and heart failure. In June 2023, the CDC Advisory Committee on Immunization Practices met to discuss recommendations for appropriate use of RSV vaccines in older adults, and outcomes are expected prior to the anticipated RSV season this fall.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





