ABSTRACT: Chronic idiopathic constipation (CIC) is a common, yet significant, disorder that can lead to impairment in a patient’s quality of life. Treatment for CIC includes dietary interventions and OTC and prescrip-tion agents to reduce worsening of the disease and to treat symptoms. Some patients may utilize OTC monotherapy, seek prescription options, or try a combination of the two before they see any improvement in symptoms. Pharmacists can help patients by providing consultation on the recommended use of each available agent.
Functional constipation describes a type of chronic constipation that does not arise from an identifiable cause or illness. Chronic idiopathic constipation (CIC) is a form of functional constipation wherein the constipation occurs spontaneously.1 CIC is a common disorder that leads to physician visits and impairment in quality of life. The diagnosis for CIC should be distinct from other gastrointestinal disorders, such as irritable bowel syndrome (IBS), opioid-induced constipation, or constipation due to dietary changes or changes in physical activity.
Diagnosis of CIC is made based on a history and physical exam of the patient.2 The Rome IV criteria provide indications for diagnosing patients with functional gastrointestinal disorders. Patients must have symptoms present for at least 3 months with an onset at least 6 months prior to diagnosis. The Rome IV criteria define a diagnosis of functional constipation with at least two or more of the following diagnostic criteria: straining, lumpy or hard stools, sensation of incomplete evacuation, sensation of anorectal obstruction/blockage, manual maneuvers to facilitate defecations, fewer than three bowel movements per week, loose stools that are rarely present without the use of laxatives, and insufficient criteria for IBS.3 Diagnosis can be confirmed through imaging studies such as colonoscopy.2
Risk factors for CIC include increased age, female gender, low-caloric diets, sedentary lifestyle, and low fiber and water intake.4 The American Gastroenterological Association and the American College of Gastroenterology (AGA/ACG) 2023 guideline on the pharmacologic management of chronic idiopathic constipation aim to provide guidance to healthcare providers on appropriate pharmacologic management in adult patients. This article will focus on the evidence-based recommendations for the management of CIC in adults and the information in the 2023 AGA/ACG guideline.5
Overview of the Guideline Updates
The 2023 American Gastroenterological Association–American College of Gastroenterology Clinical Practice Guideline: Pharmacological Management of Chronic Idiopathic Constipation provides official recommendations on the treatment options available for CIC, a type of functional constipation. The guideline was generated based on systematic reviews of the various OTC and prescription options, and recommendations were presented after a literature search. The recommendations were developed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. Ten recommendations were made, and these are classified based on the strength of the recommendation and certainty of evidence.5 The medications that hold strong recommendations include polyethylene glycol, bisacodyl, sodium picosulfate, lubiprostone, linaclotide, prucalopride, and plecanatide. The medications that hold conditional recommendations include fiber, magnesium oxide, lactulose, and senna.
The 2023 AGA/ACG guideline on CIC is an update to the 2013 American Gastroenterological Association Medical Position Statement on Constipation.6 The 2023 AGA/ACG guideline provides the following updated information:
• Recommendations are given for CIC rather than recommendations for generalized chronic constipation.
• No recommendations are given on physical exams before starting drug therapy, anorectal testing if drug therapy is not adequate, or pelvic floor retaining for defecatory disorders. Previously, these held strong recommendations in the Medical Position Statement.
• Lubiprostone and linaclotide now hold strong recommendations to be used as a replacement or as an adjunct to OTC agents. In the 2013 Medical Position Statement, lubiprostone and linaclotide were weak recommendations to be used in patients with normal-transit or slow-transit constipation.
• At the time of the 2013 Medical Position Statement, prucalopride and plecanatide were not approved in the United States. Therefore, there were no recommendations for prucalopride or plecanatide until the 2023 AGA/ACG guideline, which gave them strong recommendations, after they gained approval for use in the United States.
Recommendations
Patients should first be evaluated for alarm features, such as gastrointestinal bleed or weight loss, and seek appropriate diagnostic testing. For patients with CIC who have no alarm features, fiber is recommended first line, particularly for patients with low dietary fiber intake. If that provides an unsatisfactory response, osmotic laxatives are recommended alone or in combination with fiber. Patients who do not have relief with fiber or osmotic laxatives may benefit from the addition of stimulant laxatives used as needed or as short-term therapy in addition to fiber and osmotic laxatives. Secretagogues and 5-hydroxytryptamine receptor 4 (5-HT4) agonist options for patients who do not respond to OTC agents may be used as a replacement or as an adjunct to OTC agents. FIGURE 1 provides guidance for the recommendations for CIC.5,7
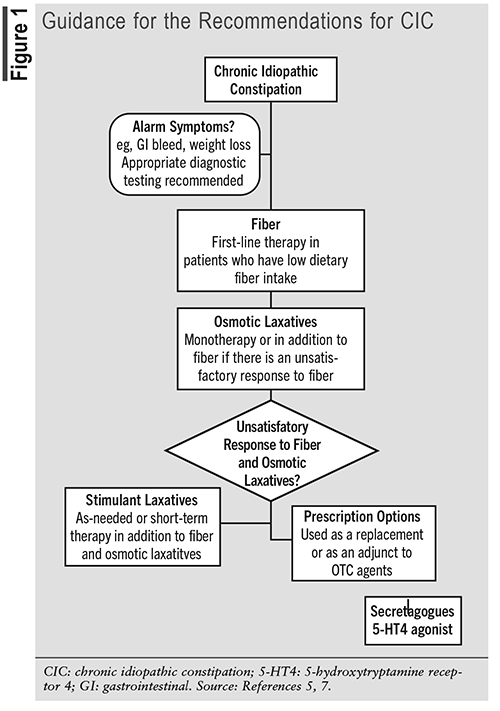
OTC and Prescription Options
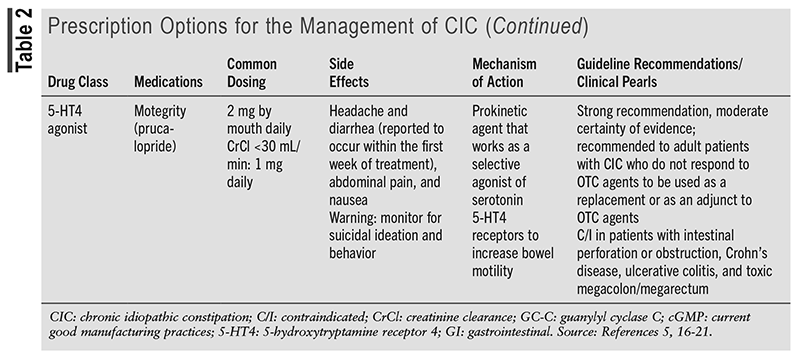
TABLE 1 outlines the OTC options for the management of CIC.5,8-16 TABLE 2 outlines the prescription options for the management of CIC.5,16-21
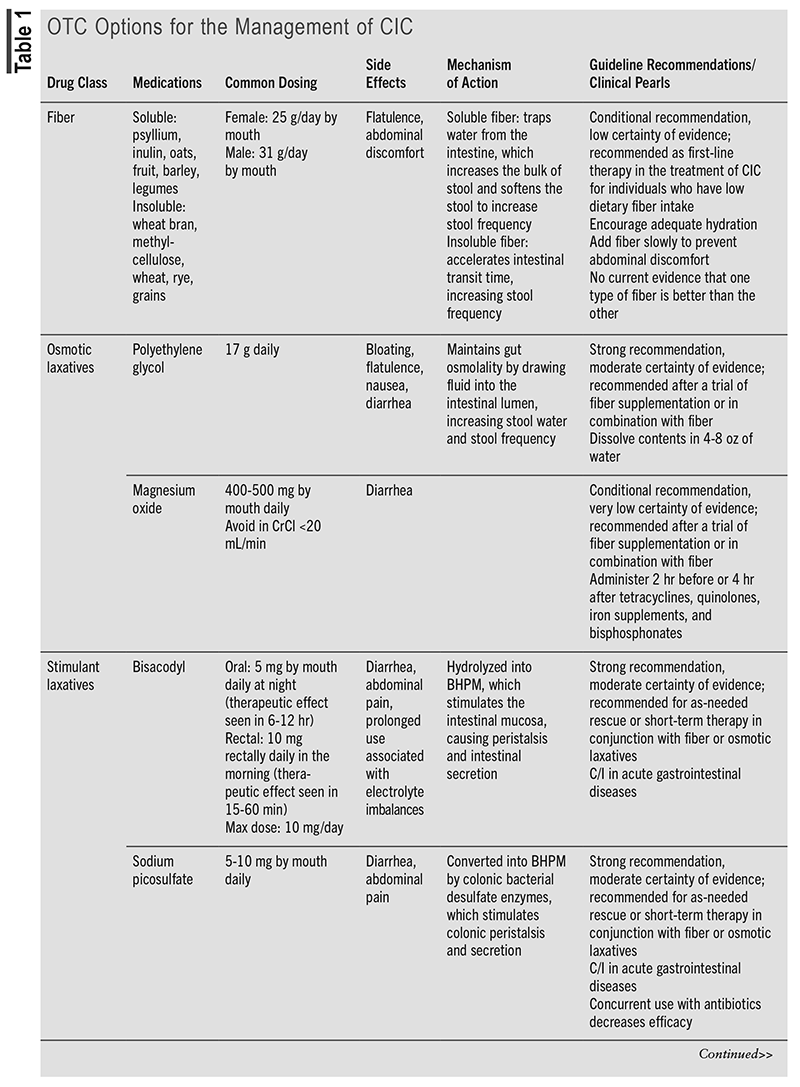
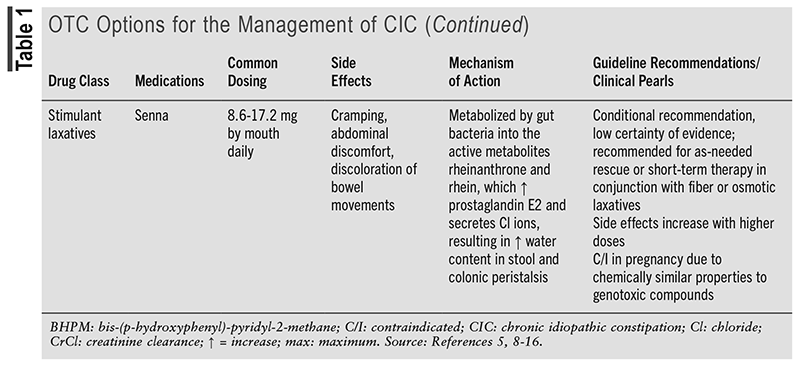
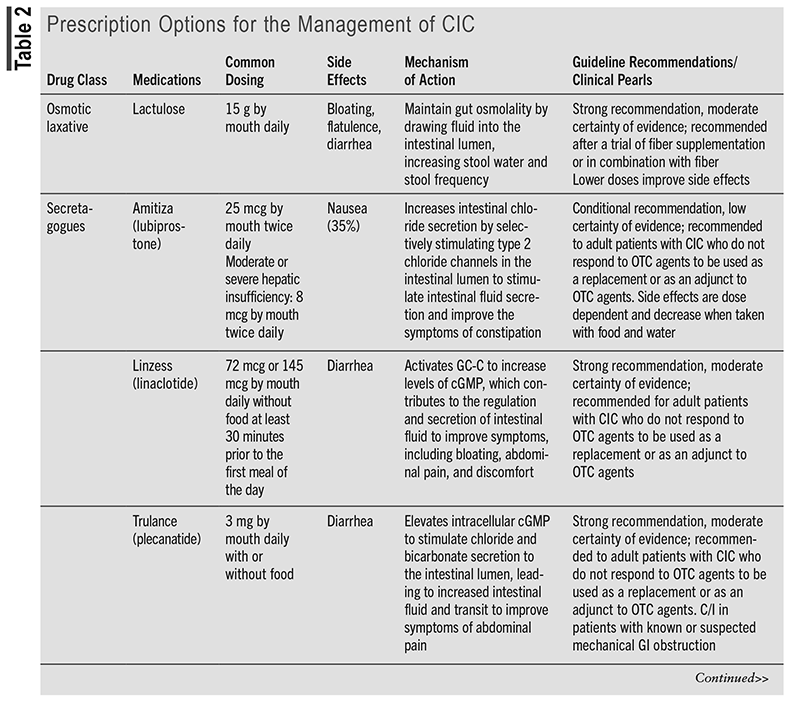
Evidence for Prescription Options
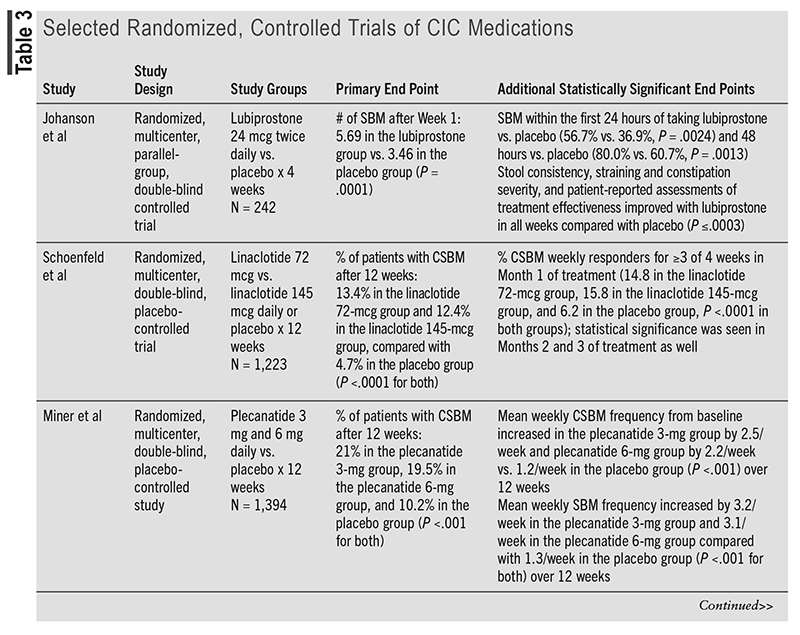
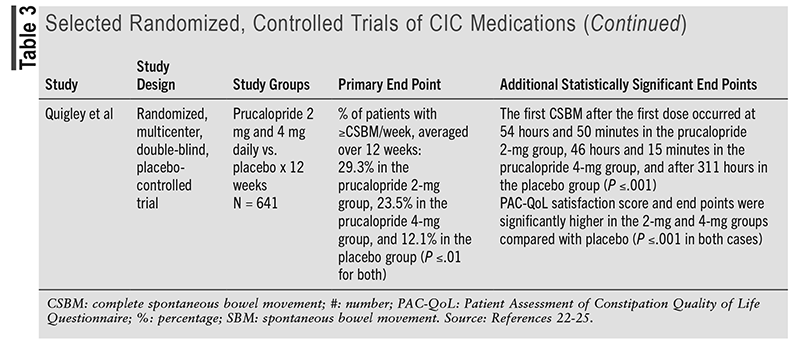
Compared with the 2013 American Gastroenterological Association Medical Position Statement on Constipation, the prescription options lubiprostone, linaclotide, plecanatide, and prucalopride have been updated to provide strong recommendations with a moderate certainty of evidence. The 2023 AGA/ACG guideline mentions various articles to support the current recommendations.5 TABLE 3 discusses one of the randomized, controlled trials mentioned in the guideline for each medication to support the current strong recommendations based on statistically significant end points.22-25
The Role of the Pharmacist
Pharmacists play a role in counseling patients on the different treatment options available for CIC. When patients are seeking recommendations, pharmacists should first evaluate a patient’s symptoms and refer as appropriate for alarm features. After identifying patients who do not require referral, community pharmacists can assist patients in locating OTC options and recommending an appropriate agent. Patients may try multiple treatments or a combination of treatments before settling on a satisfactory option.
A limitation of the available prescription options may be cost. The secretagogues, including Linzess (linaclotide) and Trulance (plecanatide), as well as the 5-HT4 agonist Motegrity (prucalopride), are only available as brand-name products. As a result, these options have a higher cost compared with the OTC options. However, the secretagogue Amitizia (lubiprostone) is now available as a generic medication and may be more cost-effective. Pharmacists can counsel patients on the use and effectiveness of both OTC and prescription options and further assist them in checking insurance coverage to address financial and insurance barriers. In addition, information on adverse events is an important point to counsel patients on when helping choose a regimen for CIC to individualize treatment, enhance long-term outcomes, and improve patient satisfaction.
Conclusion
The 2023 AGA/ACG guideline on the pharmacologic management of CIC aims to provide guidance to healthcare providers on appropriate pharmacologic management in adult patients. The treatment for CIC includes OTC and prescription options. Patients may start with a trial of fiber supplementation with or without osmotic laxatives. Stimulant laxatives may be used as needed or as short-term therapy in combination with fiber or osmotic laxatives. Prescription options are reserved if OTC agents do not provide satisfactory relief either as monotherapy or as an adjunct to OTC agents. Pharmacists may assist patients by providing consultation on the available options, including the indication, benefit, and potential side effects, and help to evaluate the cost of the various recommendations.
REFERENCES
1. Jamshed N, Lee Z-E, Olden KW. Diagnostic approach to chronic constipation in adults. Am Fam Physician. 2011. www.aafp.org/pubs/afp/issues/2011/0801/p299.html. Accessed August 1, 2023.
2. Mandal A. What is chronic idiopathic constipation (CIC)? [Internet]. 2023. www.news-medical.net/health/What-is-Chronic-Idiopathic-Constipation-(CIC).aspx. Accessed September 4, 2023.
3. Rome IV criteria [Internet]. 2023 [cited 2023 Sept 4]. https://theromefoundation.org/rome-iv/rome-iv-criteria/. Accessed September 4, 2023.
4. A treatment option [Internet]. www.motegrity.com/cic. Accessed September 4, 2023.
5. Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology Clinical Practice Guideline: Pharmacological Management of Chronic Idiopathic Constipation. Gastroenterology. 2023;16.
6. Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):P211-P217.
7. Pharmacological management of chronic idiopathic constipation [Internet]. https://gastro.org/clinical-guidance/pharmacological-management-of-chronic-idiopathic-constipation-cic/. Accessed September 13, 2023.
8. Gordon B. Nutrition tips for relieving constipation [Internet]. Chicago: Academy of Nutrition and Dietetics; 2020 [Updated 2023 Sep 4]. www.eatright.org/health/health-conditions/digestive-and-gastrointestinal/nutrition-tips-for-relieving-constipation.
9. Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011;33(8):895-901.
10. Bharucha AE, Wald A. Chronic constipation. Mayo Clin Proc. 2019;94(11):2340-2357.
11. Dabaja A, Dabaja A, Abbas M. Polyethylene glycol. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [Upated 2023 May 8]. www.ncbi.nlm.nih.gov/books/NBK557652/.
12. Mori H, Tack J, Suzuki H. Magnesium oxide in constipation. Nutrients. 2021;13(2):421.
13. Lawrensia S, Raja A. Bisacodyl. [Updated 2022 Oct 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. www.ncbi.nlm.nih.gov/books/NBK547733/.
14. Portalatin M, Winstead N. Medical management of constipation. Clin Colon Rectal Surg. 2012;25(1):12-19.
15. Sennosides [Internet]. Drug Bank Online; 2016 [Updated 2023 Sept 4]. https://go.drugbank.com/drugs/DB11365.
16. Mukherjee S, John S. Lactulose. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [Updated 2022 Jul 11]. www.ncbi.nlm.nih.gov/books/NBK536930/.
17. Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic constipation. Clin Interv Aging. 2008;3.
18. Thomas RH, Allmond K. Linaclotide (Linzess) for irritable bowel syndrome with constipation and for chronic idiopathic constipation. Pharm Times. 2013;38(3):154-60.
19. Kamuda JA, Mazzola N. Plecanatide (Trulance) for chronic idiopathic constipation and irritable bowel syndrome with constipation. Pharm Times. 2018;43(4):207-232.
20. Motegrity (prucalopride) prescribing information. www.centerwatch.com/directories/1067-fda-approved-drugs/listing/3847-motegrity-prucalopride. Accessed August 25, 2023.
21. FDA. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210166s000lbl.pdf. Accessed August 25, 2023.
22. Johanson JF, Morton D, Geenen J, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol. 2008;103.
23. Schoenfeld P, Lacy BE, Chey WD, et al. Low-dose linaclotide (72 μg) for chronic idiopathic constipation: a 12-week, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2018;113.
24. Miner PB Jr, Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017;112.
25. Quigley EM, Vandeplassche L, Kerstens R, et al. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation—a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





