US Pharm. 2022;47(8):5-12.
ABSTRACT: Loaded teas, a recent health-and-fitness trend, are visually appealing beverages with clever names that attract young consumers. There is no standard recipe, but these products contain caffeine, the amount of which varies considerably between products and often exceeds the maximum daily recommended amount for children and adolescents. Heavily marketed on social media, loaded teas are sold as nutritional supplements, thus bypassing FDA regulation. Despite limited clinical data on the health risks of loaded teas, information can be extrapolated from research on caffeine-laden energy drinks. Greater awareness and education about the potential adverse health effects of loaded-tea consumption are crucial in preventing harm to youths.
Loaded teas, which have been on the consumer market since the early 2000s, have recently surged in popularity. These cocktails of stimulants; vitamins, antioxidants, and other supplements; plant extracts; artificial sweeteners; sugar alcohols; syrups; fruit juices; and food colorings and flavorings are touted as the perfect low-calorie, sugar-free beverage for fitness enthusiasts seeking to suppress appetite, speed up metabolism, burn fat, lose weight, increase energy, enhance performance, and boost the immune system.1-4 These trendy, cleverly named, visually appealing drinks are glamorized on social media and are attractive to young consumers.3 The most popular producer of loaded-tea supplements is Herbalife Nutrition, a multilevel marketing company that generates profits from independent distributors who sell their products at locally owned nutrition shops.1,3 These shops are opening up across the country at an astonishing rate. Loaded-tea kits for at-home preparation are also available.3 Because loaded teas are considered a food supplement, they are not regulated by the FDA and distributors are not required to fully disclose the ingredients.1
Many components of loaded teas, such as caffeine, guarana, ginseng, taurine, inositol, and niacin, are also found in energy drinks.1,2,4 There is no standard recipe, however, and ingredient types and amounts vary considerably. In fact, some loaded teas may not actually contain tea. This ambiguity poses health risks given that several of these ingredients interact with prescription medications. Along with the increased prevalence of nutrition shops and social media advertising, the number of children and adolescents who drink loaded teas is rising at an alarming rate. Easy access and the absence of sales and marketing regulation are contributing factors. The consumption of energy drinks—which also contain high levels of caffeine—by the pediatric population is correlated with adverse neurologic, endocrinologic, cardiovascular (CV), gastrointestinal (GI), and behavioral effects as well as an increase in emergency department visits.5-8 Given the similarities between loaded teas and energy drinks, these adverse effects (AEs) could also occur with loaded teas. This article will describe loaded teas, discuss potential health dangers, and highlight the pharmacist’s role in educating youths and parents about them.
Overview of Loaded Teas
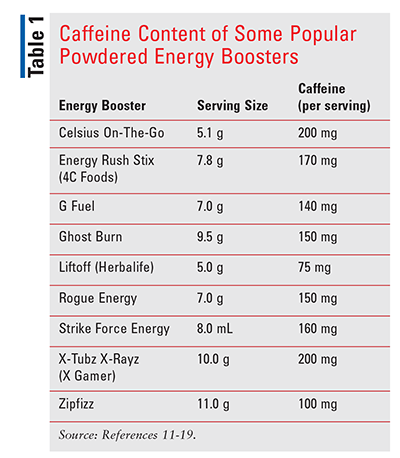
Ingredients: The term “loaded tea” is misleading, as these beverages often are not a true tea product; rather, they are a combination of powdered herbal-tea extracts, a caffeine-packed sugar-free booster, and water. Irrespective of the formulation, the average loaded tea provides little nutritional value—approximately 25 calories per 32-oz serving. Nevertheless, these products have gained popularity thanks to flashy marketing by nutrition shops. The combination of powdered herbal-tea extracts and a booster yields a concoction similar to energy drinks: caffeine, niacin, ginseng, guarana, and high doses of vitamins B6, B12, and C. Two popular powdered tea products are Herbalife’s Herbal Tea Concentrate and Waka’s Kenyan & Chinese Green Instant Tea, which contain 85 mg and 30 mg of caffeine, respectively, per serving (1/2 tsp powder).9,10 Caffeine-packed energy boosters vary in their caffeine content per serving (TABLE 1).11-19 Combining an herbal-tea powder with an energy-boosting powder produces a loaded tea with a caffeine content of 100 mg to 285 mg per serving. For reference, the average cup of coffee contains about 95 mg of caffeine.20
Marketing and Regulation: Loaded teas contain ingredients that are considered dietary supplements. This FDA classification allows manufacturers to label supplement products with claims about their effects as long as the following disclaimer is included: “These statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.”21 Most energy-boosting powders contain a proprietary blend of ingredients purported to sharpen mental focus, maximize endurance, and reduce mental fatigue. Under the Dietary Supplement Health and Education Act (DSHEA) of 1994, dietary supplements are held to labeling requirements similar to those for food nutrition labels.22 However, proprietary supplements are obliged to list only the total quantity of the blend. Because information on individual ingredient quantities is not required, many herbal supplements are marketed with verbiage that may hide information about quantities that could be harmful.22 Under the DSHEA, FDA regulations on purity also do not apply to dietary supplements.21 Alarmingly, there is no guarantee that what is declared on the label accurately reflects what is in the supplement.
Health Risks
The maximum recommended daily intake of caffeine in children (2.5 mg/kg-6 mg/kg) and adolescents (100 mg) is significantly lower than that in adults (400 mg).8 Up to 50% of adolescents drink caffeinated energy drinks, and it is plausible that they may also consume loaded teas.5 Although clinical data regarding the potential health risks of loaded teas are just starting to emerge, substantial evidence exists on energy drinks. Similar to energy drinks, the chief AEs of loaded teas could be attributable to caffeine intoxication, a clinical syndrome that may include some or all of the following: increased heart rate (HR), cardiac arrhythmias, insomnia (sometimes accompanied by inattentiveness), tremor, seizures, hallucinations, nervousness, excitability, agitation, restlessness, mood changes, facial flushing, increased body temperature, GI disturbances, diuresis, kidney failure, or even death.6,23 Alarmingly, these signs and symptoms can also occur with low-dose caffeine intake (<200 mg) in vulnerable populations, such as children, elderly persons, and individuals who are psychologically stressed, mentally ill, or caffeine-naïve.
Additional physiological and behavioral AEs, including addiction and dependence, are elicited by other loaded-tea ingredients.8 Given the considerable growth that occurs during childhood and adolescence, potential effects on brain development should also be considered, and little is known about the chronic, long-term health risks of exposure to caffeine and other loaded-tea ingredients during growth and development.24 Youths typically have smaller bodies, and they have not developed pharmacologic tolerance to stimulants.24 Accidental ingestion by very young children is another cause for concern.25 Energy drink–related visits to emergency departments by adolescents aged 12 to 17 years are rising, with more than 50% of visits attributable to energy drink ingestion alone (vs. in combination with other substances, such as alcohol); calls to poison control centers are also increasing.25-27 Given the relative ease of access and the lack of FDA regulation, similar trends may develop with loaded teas.
Neuroendocrine: Functioning of the hypothalamic-pituitary-adrenal (HPA) axis—the principal neuroendocrine system controlling bodily responses to environmental (physiological and psychological) stressors—and stress responsiveness change considerably during adolescence.28 Puberty is a critical phase of brain and neuroendocrine development and maturation during which hormonal responses to stress become heightened.28-30 Regulation of the HPA axis is mediated at least in part by estrogen and testosterone, which surge during puberty.29,31 Moreover, the anterior pituitary gland produces growth hormone (GH), thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone, and adrenocorticotropic hormone (ACTH), which are essential for healthy peripubertal development. ACTH targets the adrenal cortex to promote the secretion of glucocorticoids (GCs) such as cortisol. Cortisol is important in the peripubertal development of neuronal plasticity, which governs lifelong responses to stress. Stress-induced HPA-axis stimulation and GC release are normally short-lived owing to negative feedback control.29
Most information about HPA-axis function during adolescence and modulation by caffeine comes from preclinical studies, which have reported developmental stage- and sex-related variations in GC secretion, anxiety responses, and adrenal anatomy. For instance, compared with adult rats, prepubertal rats displayed prolonged GC release in response to stress.29 Along with prolonged exposure to chronic stress, caffeine chronically activates the HPA axis.32 Furthermore, caffeine consumption may disrupt HPA axis development and impair stress responses during development.30 In peripubertal rats, high-dose caffeine affected the microarchitecture and reduced adrenal-gland size in females, reduced GC production in males and females, and increased ACTH levels. Caffeine withdrawal following month-long consumption may have long-lasting effects, including heightened anxiety-related behavior, as observed in adolescent versus adult rodents.33 These data suggest that juvenile consumption of high-dose caffeine may increase the risk of HPA dysregulation, which is associated with mood disorders (e.g., depression, anxiety), immune-system dysfunction, and CV disease.34
Regarding developmental growth, preclinical data indicate that several ingredients in loaded teas target insulin-like growth factor-1 (IGF-1) and GH. In prepubertal rats, chronic caffeine consumption lowered IGF-1 levels, delayed bone growth and maturation, and possibly suppressed ossification.35 Ginseng increased serum GH levels and gene expression in rat pituitary and brain.36 Taurine was found to stimulate the IGF1R/ERK intracellular signaling pathway and increase osteoblast proliferation in vitro.37
In terms of neurotransmission, caffeine increased dopamine, gamma-aminobutyric acid, and glutamate levels in juvenile rats.38 Taurine increased dopamine in brain circuits involved in reward, and ginseng activated brain dopamine utilization in young rats.39,40 Energy drinks have been found to increase dopamine release in brain reward circuitry and to stimulate dopamine transmission in a manner similar to that observed with drugs of abuse.41 Also, taurine-induced increases in dopamine are similar to those observed with ethanol.41 It is feasible that loaded tea–evoked dopamine release in the brain can lead to craving, compulsive seeking, and addiction.41 Resting plasma norepinephrine levels are also elevated by energy drinks and caffeine.42,43 Rb3, a ginsenoside and main chemical component of ginseng, selectively alters brain norepinephrine levels in a site-dependent and dose-dependent fashion.44 The overall elevation of norepinephrine elicited by loaded-tea components is consistent with the increased sympathetic drive and blood pressure (BP) accompanying energy-drink ingestion.
Cardiometabolic: Despite a paucity of clinical data, potential cardiometabolic dangers of loaded teas in youths can be extrapolated from information on energy drinks. In healthy children and adolescents, acute intake of high-dose caffeine via energy drinks is associated with reduced HR, increased systolic and diastolic BP, increased arterial stiffness, arrythmia, reduced cerebral blood flow, and hyperglycemia with hyperinsulinemia.45-48 The precise profile of the cardiometabolic response to caffeine is influenced by sex and pubertal stage, with significant sex differences observed in postpubertal youth and caffeine-mediated effects related to menstrual-cycle phases in girls.49,50 Age- and sex-dependent effects of caffeine may also be influenced by dose as well as genetic and environmental interactions.49,50
Public perception that the consumption of high-calorie energy drinks can cause childhood obesity and diabetes could lead to the erroneous assumption that low-calorie loaded teas are healthier.5 Children and adolescents with eating disorders (e.g., anorexia nervosa) typically present with low BP and poor circulation and consume excessive caffeine to offset calorie deficit–induced fatigue, suppress appetite, loosen stools, and promote diuresis. In this context, the marketing of loaded tea as an appetite suppressant could be problematic because caffeine causes electrolyte imbalances (i.e., hypocalcemia and hypokalemia) and accordingly could exacerbate underlying electrolyte disorders and heighten the risk of cardiac morbidity and mortality.5
When caffeine and taurine are consumed together (as is common with loaded teas), cardiac effects can be synergistic, leading to more pronounced changes in HR and systolic BP.5,51 These cardiometabolic AEs may be worse in youths with preexisting conditions, and patients with cardiovascular comorbidities (e.g., ion channelopathies, hypertrophic cardiomyopathy) are especially susceptible to hypertension, syncope, arrhythmias, and even sudden death.5 Children with attention-deficit/hyperactivity disorder (ADHD) who are taking other stimulants and adolescents who are experiencing rapid growth phases may be at particular risk.5
GI: The primary ingredient in loaded teas is caffeine, which is rapidly absorbed (30-60 minutes) from the GI tract and is converted to paraxanthine (primarily), theobromine, and theophylline.8 Following rapid and complete absorption in the small intestine, caffeine quickly diffuses to other tissues.26 Caffeine is both water-soluble and lipid-soluble, so it readily crosses the blood-brain barrier and is found in virtually every bodily fluid.26 As a stimulant, caffeine stimulates gastrin release and gastric-acid secretion.52 It stimulates gut motility (i.e., peristalsis) by increasing colonic motor activity.52 Caffeine also increases anal-sphincter pressure and strengthens the force of sphincter contraction to accelerate a bowel movement.53 These GI-stimulatory effects may loosen stools, resulting in diarrhea. GI-distress symptoms of caffeine intoxication are upset stomach, abdominal cramping, nausea or vomiting, diarrhea, and dehydration.5,54
Behavioral: Socioemotional effects of caffeine intake include sleep disturbances, daytime sleepiness, irritability, anger, anxiety, restlessness, getting into trouble (e.g., violence, conduct disorders), and risky behavior.7,55-59 Adolescents who consume caffeinated drinks are more likely to report nicotine and alcohol use.7,60 Adolescent consumption of energy drinks has been associated with smoking and use of alcohol and illicit drugs, and teens often combine energy drinks with alcohol.7,58,61 Being a stimulant, caffeine masks the depressant effects of alcohol, giving individuals who mix them the false perception that they are more sober than they really are, and the masking of alcohol intoxication can increase risk behaviors.58,62,63 Caffeine also provides the individual with the energy to drink excessive alcohol, increasing the likelihood of needing emergency care.64
Potential Drug Interactions: Pharmacists should be prepared to counsel patients and caregivers on potential interactions between loaded-tea ingredients and medications commonly used in children and adolescents. The caffeine in loaded teas can interact with several frequently prescribed medications. Central nervous system (CNS) stimulants (e.g., amphetamine-dextroamphetamine, lisdexamfetamine, methylphenidate, dexmethylphenidate, modafinil, armodafinil) are often used in patients with ADHD or narcolepsy. Excessive consumption of caffeine should be avoided with concomitant use of CNS stimulants, as patients can experience additive CNS-stimulating effects such as anxiety, irritability, tremors, insomnia, and nausea.65 Bupropion, which may be used off-label in selected pediatric and adolescent patients with ADHD, can lower the seizure threshold in a dose-dependent manner, and concomitant use of caffeine can increase the risk of seizures. Pharmacists should advise these patients to limit their caffeine intake while on bupropion therapy.65,66
Lithium may be used as a mood-stabilizing agent in patients with bipolar disorder. Caffeine can decrease the serum lithium concentration, which can be problematic while a patient is being stabilized on an effective dose. Given lithium’s narrow therapeutic index, pharmacists should caution patients that their intake of caffeine (e.g., coffee, cola, green tea) can affect how the lithium is dosed. Increased caffeine intake can negatively affect the efficacy of lithium therapy, and an abrupt reduction of caffeine intake increases the risk of lithium-associated AEs (e.g., nausea, vomiting, diarrhea, drowsiness, tremors).65,67
In patients with obsessive-compulsive disorder or generalized anxiety disorder, the selective serotonin reuptake inhibitor fluvoxamine may be prescribed. As a CYP1A2 inhibitor, fluvoxamine can decrease hepatic caffeine metabolism, thereby increasing the risk of caffeine-associated AEs. Patients should be advised to limit consumption of caffeine, as it can worsen anxiety symptoms.65,68
In asthma patients, theophylline may be used as an alternative maintenance agent to control symptoms. Patients should be advised to limit caffeine intake, as concomitant caffeine and theophylline therapy can result in increased caffeine-associated AEs, including nausea, anxiety, irritability, tremors, and insomnia.65,69
Pharmacist Counseling Points
Given the potential health risks of loaded-tea consumption in youth, it is incumbent upon practitioners to raise awareness through education. Focusing on the most relevant counseling points will increase the effectiveness of these conversations. The RUN method (Read Labels, Understand Interactions, Notice Signs/Symptoms) may be a useful tool for parents and adolescents to foster responsible consumption of loaded teas. The first principle, Read Labels, is a foundational step. It is important to know not only what ingredients are being consumed, but also how much of each. Caffeine intake in children and adolescents should not exceed the maximum recommended daily amount of 2.5 to 6 mg/kg and 100 mg, respectively. Parents should also be cognizant of herbal ingredients, such as ginseng and taurine, that can exhibit effects similar to those of caffeine. Ginseng, in particular, has multiple potential drug interactions.8
The second principle, Understand Interactions, involves educating parents about not only the stimulating effects of caffeine, but also its synergistic effects with other substances. Parents should be made aware of the effects of combining caffeine with prescription stimulant medications, alcohol, and herbal ingredients. Understanding interactions is particularly relevant when a child is taking stimulant medications to treat ADHD.
The third principle, Notice Signs/Symptoms, creates an opportunity for parents to readily identify potential health concerns. The high caffeine content of loaded teas makes caffeine intoxication, particularly CV sequelae, a primary focus. Changes in BP and HR are pertinent signs to note. Nausea, anxiety, agitation, and seizures may also be features of caffeine intoxication.54 Additionally, parents should be attentive to mood changes, facial flushing, and insomnia. The overarching theme of the RUN method is awareness. Increased public awareness can lead to greater vigilance and significantly impact health decisions.
REFERENCES
1. Landsverk G. Colorful ‘loaded teas’ are filling up Instagram, pitched as a ‘clean’ energy drink. Nutritionists are not impressed. Insider. www.insider.com/what-is-loaded-tea-is-it-safe-healthy-2020-9. Accessed April 25, 2022.
2. Frishberg H. What is ‘loaded tea’ and will it land you in the emergency room? New York Post. https://nypost.com/2020/08/19/what-is-loaded-tea-and-will-it-land-you-in-the-emergency-room/. Accessed April 25, 2022.
3. McCarthy A. The multilevel truth behind small town America’s latest tea obsession. Eater. www.eater.com/22958985/loaded-teas-herbalife-mlm-silver-lining-lessons-dupes-nutrition-clubs. Accessed April 25, 2022.
4. Capritto A. The dangers of loaded tea: how much caffeine is too much? www.cnet.com/health/nutrition/is-loaded-tea-good-or-bad-for-you/. Accessed April 25, 2022.
5. Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127:511-528.
6. Temple JL. Review: trends, safety, and recommendations for caffeine use in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2019;58:36-45.
7. Harris JL, Munsell CR. Energy drinks and adolescents: what’s the harm? Nutr Rev. 2015;3:247-257.
8. De Sanctis V, Soliman N, Soliman AT, et al. Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: a significant public health hazard. Acta Biomed. 2017;88:222-231.
9. Herbalife Nutrition. Herbal Tea Concentrate Original. www.herbalife.com/products/herbal-tea-concentrate-original-1oz/#product-overview. Accessed May 19, 2022.
10. Waka. Kenyan & Chinese Green Instant Tea 4.5 oz Bag. www.wakacoffee.com/products/kenyan-chinese-green-instant-tea-4-5-oz-bag?_pos=4&_fid=c3c59b854&_ss=c. Accessed May 19, 2022.
11. Celsius. Celsius On-The-Go Berry. www.celsius.com/products/on-the-go/berry/. Accessed May 19, 2022.
12. 4C Foods. Caffeine content. www.4c.com/caffeine-content/. Accessed May 19, 2022.
13. G Fuel. G Fuel Energy Formula Star Fruit. https://gfuel.com/collections/tubs/products/star-fruit-tub. Accessed May 19, 2022.
14. Ghost. Ghost Burn. www.ghostlifestyle.com/products/ghost-burn?variant=39748946395313. Accessed May 19, 2022.
15. Herbalife Nutrition. Liftoff. www.herbalife.com/our-products/energy-fitness/energy-supplements/. Accessed May 19, 2022.
16. Rogue Energy. Rogue Energy Black Cherry. https://rogueenergy.com/products/black-cherry-energy. Accessed May 19, 2022.
17. Strike Force Energy. Nutrition. www.strikeforceenergy.com/pages/nutrition. Accessed May 19, 2022.
18. X-Gamer. X-Tubz X-Rayz (600g/60 servings). https://x-gamer.com/collections/x-tubz/products/x-tubz-x-rayz-600g-60-servings. Accessed May 19, 2022.
19. Zipfizz. Zipfizz Drink Mix. https://zipfizz.com/products/powders. Accessed May 19, 2022.
20. FoodData Central. Coffee, brewed. https://fdc.nal.usda.gov/fdc-app.html#/food-details/1104137/nutrients. Accessed May 19, 2022.
21. FDA. Dietary supplement labeling guide: chapter VI. Claims. www.fda.gov/food/dietary-supplements-guidance-documents-regulatory-information/dietary-supplement-labeling-guide-chapter-vi-claims. Accessed May 19, 2022.
22. National Institutes of Health Office of Dietary Supplements. Dietary Supplement Health and Education Act of 1994. https://ods.od.nih.gov/About/dshea_Wording.aspx. Accessed May 19, 2022.
23. Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33:793-806.
24. Curran CP, Marczinski CA. Taurine, caffeine, and energy drinks: reviewing the risks to the adolescent brain. Birth Defects Res. 2017;109:1640-1648.
25. Seifert SM, Seifert SA, Schaechter JL, et al. An analysis of energy-drink toxicity in the National Poison Data System. Clin Toxicol (Phila). 2013;51:566-574.
26. Temple JL, Bernard C, Lipshultz C, et al. The safety of ingested caffeine: a comprehensive review. Front Psychiatry. 2017;8:80.
27. Ruiz LD, Scherr RE. Risk of energy drink consumption to adolescent health. Am J Lifestyle Med. 2019;13:22-25.
28. Romeo RD. The teenage brain: the stress response and the adolescent brain. Curr Dir Psychol Sci. 2013;22:140-145.
29. McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220-233.
30. Ryu KY, Roh J. The effects of high peripubertal caffeine exposure on the adrenal gland in immature male and female rats. Nutrients. 2019;11:951.
31. Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113-132.
32. Patz MD, Day HEW, Burow A, Campeau S. Modulation of the hypothalamo-pituitary-adrenocortical axis by caffeine. Psychoneuroendocrinology. 2006;31:493-500.
33. O’Neill CE, Newsom RJ, Stafford J, et al. Adolescent caffeine consumption increases adulthood anxiety-related behavior and modifies neuroendocrine signaling. Psychoneuroendocrinology. 2016;67:40-50.
34. Sheng JA, Bales NJ, Myers SA, et al. The hypothalamic-pituitary-adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Front Behav Neurosci. 2021;14:601939.
35. Shin J, Choi Y, Kim J, et al. High doses of caffeine reduce in vivo osteogenic activity in prepubertal rats. J Anat. 2015;227:10-20.
36. Yoshizato H, Fujikawa T, Shibata M, et al. Stimulation of growth hormone gene expression in the pituitary and brain by Panax ginseng C. A. MEYER. Endocr J. 1999;46 suppl:S85-S88.
37. Roman-Garcia P, Quiros-Gonzalez Y, Mottram L, et al. Vitamin B12-dependent taurine synthesis regulates growth and bone mass. J Clin Invest. 2014;124:2988-3002.
38. Owolabi JO, Olatunji SY, Olanrewaju AJ. Caffeine and cannabis effects on vital neurotransmitters and enzymes in the brain tissue of juvenile experimental rats. Ann Neurosci. 2017;24:65-73.
39. Ericson M, Molander A, Stomberg R, Söderpalm B. Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. Eur J Neurosci. 2006;23:3225-3229.
40. Watanabe H, Ohta H, Imamura L, et al. Effect of Panax ginseng on age-related changes in the spontaneous motor activity and dopaminergic nervous system in the rat. Jpn J Pharmacol. 1991;55:51-56.
41. Vargiu R, Broccia F, Lobina C, et al. Chronic Red Bull consumption during adolescence: effect on mesocortical and mesolimbic dopamine transmission and cardiovascular system in adult rats. Pharmaceuticals (Basel). 2021;14:609.
42. Svatikova A, Covassin N, Somers KR, et al. A randomized trial of cardiovascular responses to energy drink consumption in healthy adults. JAMA. 2015;314:2079-2082.
43. Lane JD, Adcock RA, Williams RB, Kuhn CM. Caffeine effects on cardiovascular and neuroendocrine responses to acute psychosocial stress and their relationship to level of habitual caffeine consumption. Psychosom Med. 1990;52:320-336.
44. Cui J, Jiang, L, Xiang H. Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. J Psychopharmacology. 2012;26:697-713.
45. Oberhoffer FS, Li P, Jakob A, et al. Energy drinks: effects on blood pressure and heart rate in children and teenagers. A randomized trial. Front Cardiovasc Med. 2022;9:862041.
46. Li P, Mandilaras G, Jakob A, et al. Energy drinks and their acute effects on arterial stiffness in healthy children and teenagers: a randomized trial. J Clin Med. 2022;11:2087.
47. Mandilaras G, Li P, Dalla-Pozza R, et al. Energy drinks and their acute effects on heart rhythm and electrocardiographic time intervals in healthy children and teenagers: a randomized trial. Cells. 2022;11:498.
48. Moussa M, Hansz K, Rasmussen M, et al. Cardiovascular effects of energy drinks in the pediatric population. Pediatr Emerg Care. 2021;37:578-582.
49. Temple JL, Ziegler AM, Graczyk A, et al. Cardiovascular responses to caffeine by gender and pubertal stage. Pediatrics. 2014;134:e112-e119.
50. Temple JL, Ziegler AM, Martin C, de Wit H. Subjective responses to caffeine are influenced by caffeine dose, sex, and pubertal stage. J Caffeine Res. 2015;5:167-175.
51. Baum M, Weiss M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids. 2001;20:75-82.
52. Nehlig A. Effects of coffee on the gastro-intestinal tract: a narrative review and literature update. Nutrients. 2022;14:399.
53. Lohsiriwat S, Kongmuang P, Leelakusolvong S. Effects of caffeine on anorectal manometric findings. Dis Colon Rectum. 2008;51:928-931.
54. Willson C. The clinical toxicology of caffeine: a review and case study. Toxicol Rep. 2018;5:1140-1152.
55. “Hearing to examine energy drinks, focusing on exploring concerns about marketing to youth.” Testimony of Marcie Beth Schneider, MD, FAAP, on behalf of the American Academy of Pediatrics before the Senate Committee on Commerce, Science, and Transportation. www.commerce.senate.gov/services/files/E22801F3-D334-4737-A54D-3189FA198BC0. Accessed May 26, 2022.
56. Kristjansson AL, Sigfusdottir ID, Frost SS, James JE. Adolescent caffeine consumption and self-reported violence and conduct disorder. J Youth Adolesc. 2013;42:1053-1062.
57. Jackson DAE, Cotter BV, Merchant RC, et al. Behavioral and physiologic adverse effects in adolescent and young adult emergency department patients reporting use of energy drinks and caffeine. Clin Toxicol (Phila). 2013;51:557-565.
58. Miller KE. Energy drinks, race, and problem behaviors among college students. J Adolesc Health. 2008;43:490-497.
59. James JE, Kristjansson AL, Sigfusdottir ID. Adolescent substance use, sleep, and academic achievement: evidence of harm due to caffeine. J Adolesc. 2011;34:665-673.
60. Kristjansson AL, Kogan SM, Mann MJ, et al. Does early exposure to caffeine promote smoking and alcohol use behavior? A prospective analysis of middle school students. Addiction. 2018;113:1706-1713.
61. Terry-McElrath YM, O’Malley PM, Johnston LD. Energy drinks, soft drinks, and substance use among United States secondary school students. J Addict Med. 2014;8:6-13.
62. Kaminer Y. Problematic use of energy drinks by adolescents. Child Adolesc Psychiatr Clinic N Am. 2010;19:643-650.
63. Arria AM, Bugbee BA, Caldeira KM, Vincent KB. Evidence and knowledge gaps for the association between energy drink use and high-risk behaviors among adolescents and young adults. Nutr Rev. 2014;72(suppl 1):87-97.
64. Bonar EE, Cunningham RM, Polshkova S, et al. Alcohol and energy drink use among adolescents seeking emergency department care. Addict Behav. 2015;43:11-17.
65. Caffeine. Clinical Pharmacology [online database]. www.clinicalkey.com/pharmacology/monograph/81?n=Caffeine. Accessed May 12, 2022.
66. Bupropion. Clinical Pharmacology [online database]. www.clinicalkey.com.com/pharmacology/monograph/76?n=Bupropion. Accessed May 12, 2022.
67. Lithium. Clinical Pharmacology [online database]. www.clinicalkey.com/pharmacology/monograph/351?n=Lithium. Accessed May 12, 2022.68. Fluvoxamine. Clinical Pharmacology [online database]. www.clinicalkey.com/pharmacology/monograph/265?n=Fluvoxamine. Accessed May 12, 2022.
69. Theophylline. Clinical Pharmacology [online database]. www.clinicalkey.com/pharmacology/monograph/599?n=Theophylline,Aminophylline. Accessed May 12, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






