US Pharm.2021;46(9):HS2-HS-6.
ABSTRACT: The COVID-19 virus has posed a serious threat to the health of individuals globally. In just over a year since the official declaration of the global pandemic, the FDA has issued emergency-use authorization of three vaccines against SARS-CoV-2, the virus responsible for COVID-19, in the United States. The third vaccine to receive this designation was from Johnson & Johnson, a single-dose adenovirus vector vaccine indicated for individuals aged 18 years and older for protection against COVID-19 infection. Since approval, the Johnson & Johnson vaccine has been associated with life-threatening thrombosis, requiring a 10-day halt in vaccine administration in April 2021. Case reports have indicated that women aged 18 to 49 years are at the highest risk for experiencing this rare but serious adverse event. Patients who experience vaccine-induced thrombotic thrombocytopenia (VITT) should be treated with nonheparin anticoagulants and IV immunoglobulin while avoiding heparin-containing products and platelet transfusions.
The extreme global impacts of the highly transmissible and sometimes fatal coronavirus (COVID-19) necessitated rapid development and implementation of a vaccine to protect against the virus. The World Health Organization officially declared COVID-19 a global pandemic on March 11, 2020. Just 9 months later, on December 11, 2020, the FDA issued the first emergency-use authorization (EUA) for the Pfizer-BioNTech vaccine in individuals aged 16 years and older for the prevention of the coronavirus. Three vaccines have now received FDA EUA, including Pfizer-BioNTech, Moderna, and Johnson & Johnson. As of June 21, 2021, 150 million people had been fully vaccinated in the United States, 53.03% of the U.S. population.1
Both the Pfizer and Moderna vaccines require a two-dose series for full-vaccination status, posing logistical hurdles to full vaccination. The FDA issuance of an EUA for the Johnson & Johnson (Janssen) vaccine on February 21, 2021, was considered a breakthrough on the road to reaching herd immunity via mass vaccination, due to its single-dose requirement.2,3 Herd immunity, which can occur through either infection or vaccination, is the resistance to the spread of COVID-19 within a population. It is important to obtain herd immunity to decrease cases and deaths within a population.
However, administration of the Johnson & Johnson vaccine was halted between April 13 and April 23, 2021, in response to six case reports of life-threatening thrombosis, all occurring in women aged 18 to 48 years.2 While the FDA has since allowed administration of the Johnson & Johnson vaccine to resume, concern still exists surrounding the risk of developing a clot following vaccination, particularly in at-risk populations. Additionally, management of this type of thrombosis differs from that of typical thrombotic etiology and is an evolving topic for discussion. This article compares the available vaccines, describes the mechanism of vaccine-induced thrombosis by the Johnson & Johnson vaccine, highlights at-risk populations, and summarizes management strategies.
Available COVID-19 Vaccines
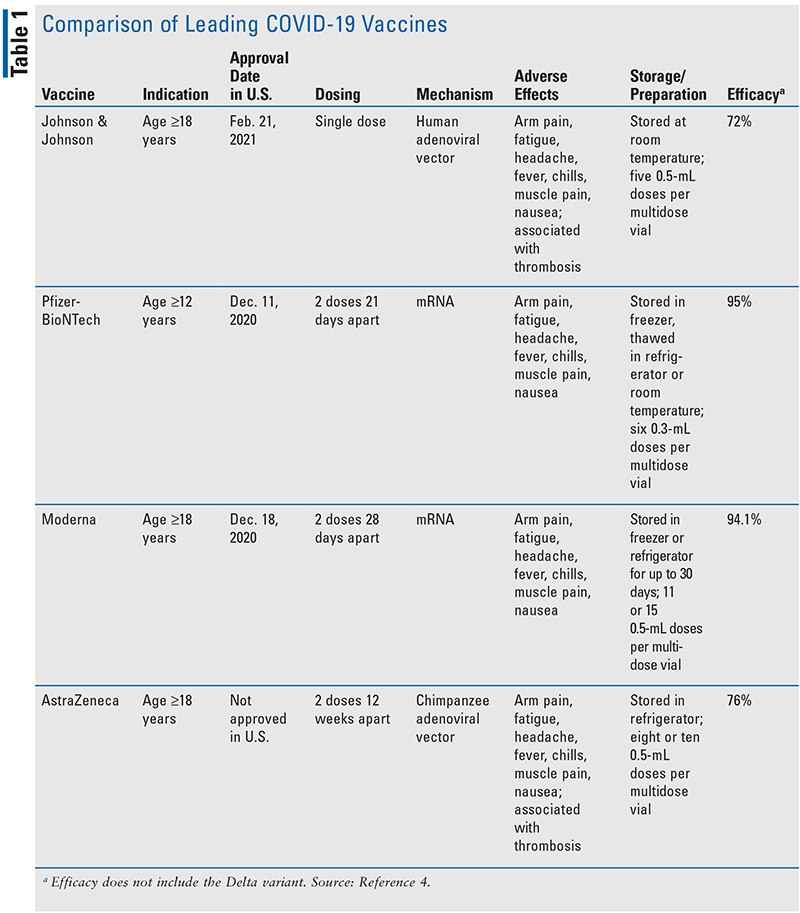
In addition to the FDA EUA for the three vaccine products in the U.S., many others are being developed and have been approved in other countries. TABLE 14 outlines the similarities and differences between the four major vaccines being used worldwide.
mRNA vs. Vector Vaccine Pharmacology
The four leading vaccines use two different mechanisms for developing immunity to SARS-CoV-2, the virus responsible for the COVID-19 disease. Pfizer-BioNTech and Moderna employ messenger RNA (mRNA) technology, while Johnson & Johnson and AstraZeneca are vector vaccines. The mRNA vaccines integrate synthesized mRNA into host cells, inducing translation of spike proteins that resemble those of SARS-CoV-2. The spike proteins are then released from the host cell and internalized by antigen-presenting cells, which elicits a humoral immune response from the host by activating B cells. Upon reexposure to the antigen, in the form of the second dose of the vaccine or exposure to the virus, memory B cells will produce an immune response sufficient to fight off the virus prior to infection.5 Advantages to mRNA technology include ease and speed of manufacturing, while a major disadvantage is storage requirements (see TABLE 1).5 The Johnson & Johnson and AstraZeneca vaccines use an inactivated adenovirus with a genetic code instructing host cells to produce a spike protein that signals the immune system to create antibodies and memory cells against SARS-CoV-2.2 While the two types of vaccines have different pharmacology, both types are effective in preventing infection with COVID-19.6
Thrombosis and the Johnson & Johnson Vaccine
Prior to the FDA pausing the Johnson & Johnson vaccination, roughly 7 million doses had been administered in the U.S.7 The CDC now estimates thrombosis following the Johnson & Johnson vaccine to occur in roughly seven individuals per every 100,000 doses given (<0.0001% risk).8 When the FDA allowed for resumed vaccination with the Johnson & Johnson vaccine following case reports of life-threatening clots in younger women, a warning was added to the label regarding a “rare but serious blood clotting disorder.” Thrombosis, in general, is a serious condition that kills over 100,000 patients in the U.S. each year.2 The specific type of clot being induced by vaccination, cerebral venous sinus thrombosis (CVST), involves a clot in the brain that prevents blood from draining out of the sinuses and can lead to intracranial hemorrhage.9 While clotting is a known complication of infection with COVID-19, neither of the other U.S.-approved vaccinations (Pfizer and Moderna), both of which are mRNA vaccines, have been associated with clotting.4 The unapproved AstraZeneca vaccine, which like Johnson & Johnson is an adenoviral vector vaccine, has been associated with the same patterns of clotting.9 This indicates that the mechanism of thrombosis is related to the mechanism of the vaccine.
The Johnson & Johnson vaccine contains a gene for a coronavirus spike protein added to an inactivated adenovirus 26, thus explaining the technical name “Ad26.COV2-S.”7 All patients in the initial reported cases of severe thrombosis following vaccination with the AstraZeneca vaccine were found to have high levels of antibodies to platelet factor 4-polyanion complexes (PF4).10 Additionally, patients with the clotting complication have displayed a prothrombotic cascade accompanied by thrombocytopenia (platelet count less than 150,000 x mm3).11 Researchers and healthcare professionals are speculating that in these rare cases of thrombosis, the antibodies that are produced to the SARS-CoV-2 spike protein are cross-reacting with PF4, similarly to the autoimmune response in heparin-induced thrombocytopenia (HIT), leading to the term vaccine-induced thrombotic thrombocytopenia (VITT).10
Acute Management of VITT
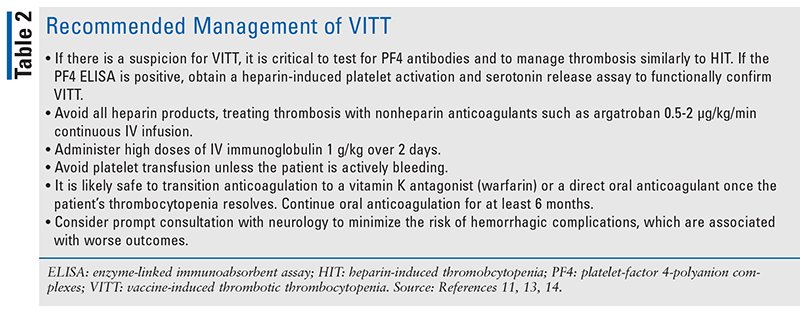
The typical patient presentation in reported cases of Johnson & Johnson–associated VITT includes new-onset severe headache within 2 to 3 weeks of vaccination, low platelet counts, significantly increased D-dimer, and low-normal fibrinogen.12 Recommended management of VITT based on case reports is described in TABLE 2.13,14
Thrombosis and COVID-19
As described above, there is a small but serious risk for developing thrombosis following vaccination with the Johnson & Johnson vaccine, particularly in women. However, it is important to also discuss the risk of thrombosis associated with infection with the COVID-19 virus. The clot risk with COVID-19 infection is approximately 100 times greater than that of vaccination with the Johnson & Johnson vaccine.12 A reported 20% of critically ill patients infected with COVID-19 develop clots.2 Coagulopathy, specifically disseminated intravascular coagulation (DIC), is a common feature of COVID-19 due to the systemic inflammatory response.15 Infected patients are at an increased risk of experiencing venous thromboembolism (VTE) due to the increased inflammatory response, DIC, immobilization, and hypoxia associated with respiratory distress.15 Hospitalized COVID-19 patients or those with risk factors for thrombosis should be initiated on VTE prophylaxis with either low-molecular-weight heparin (LMWH) or unfractionated heparin. In the case of VTE development, the clot should be treated with LMWH initially, and the patient should be transitioned to direct oral anticoagulants upon discharge.15
At-Risk Populations
Prior to April 21, 2021, 12 cases of CVST following the Johnson & Johnson vaccine had been reported. All the cases were female patients aged 18 to 60 years who presented 6 to 15 days postvaccination. None of the patients had prior exposure to heparin, and all who were tested for the PF4 antibody (11 of the 12) yielded positive results.16 Additionally, CVST disproportionately affects women younger than age 50 years, and risk factors include previous pregnancy and oral contraception.9 While there have been case reports of COVID-19 vector vaccines causing thrombosis in male patients, the overwhelming majority have been female. The CDC indicates that women aged 18 to 49 years should be aware of the risk of developing a clot following the Johnson & Johnson vaccine. Patients within this population should be able to identify the signs and symptoms of VITT, including severe headache or blurred vision, shortness of breath, chest pain, and leg swelling.16
Pharmacist’s Role
Pharmacists are in a unique position when it comes to vaccinations against COVID-19. While it is important to be aware of the clotting risk associated with the Johnson & Johnson vaccine, particularly in younger women, there is also an opportunity to educate on the risks versus benefits of receiving vaccination. There is a much higher risk of thrombosis associated with hospitalization due to COVID-19 infection than there is with vaccination, as the inflammatory response associated with COVID-19 infection induces coagulopathy.12 Pharmacists can continue to recommend the Johnson & Johnson vaccine in patients where it is indicated, including women aged 18 to 60 years. Pharmacists can educate patients on symptoms to monitor and advise patients to seek medical attention if symptoms occur. Adverse effects of any of the vaccines should be reported to the Vaccine Adverse Event Reporting System. Additionally, pharmacists in the inpatient setting can help with management of VITT patients by avoiding heparin and platelet infusions.
Conclusion
The Johnson & Johnson vaccine is a useful tool for working toward herd immunity to COVID-19 through vaccination due to its single-dose regimen and practical storage requirements. The vaccine’s association with life-threatening thrombosis is likely VITT caused by the antibodies produced via the vector vaccine mechanism. While general management of thrombosis involves heparin and typical thrombocytopenia involves platelet transfusion, VITT should be treated similarly to HIT, and therefore both heparin and platelets should be avoided. While the Johnson & Johnson vaccine does carry a risk of clotting, particularly in younger women, the risk of developing a clot secondary to COVID-19 infection is significantly higher, and the benefits of vaccination outweigh the risk in the majority of patients. Pharmacists in a variety of practice settings can be influential in ensuring patients are properly educated on the risks of thrombosis and that VITT is appropriately managed and monitored.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Coronavirus (COVID-19) vaccinations. OurWorldInData.org. Updated June 21 , 2021. https://ourworldindata.org/covid-vaccinations?country=USA. Accessed June 21, 2021.
2. Katella K. The Johnson & Johnson vaccine and blood clots: what you need to know. Yale Medicine. April 23, 2021. www.yalemedicine.org/news/coronavirus-vaccine-blood-clots. Accessed June 14, 2021.
3. Shay DK, Gee J, Su JR, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine—United States, March-April 2021. Morb Mortal Wkly Rep. 2021;70(18):680-684.
4. CDC Different COVID-19 vaccines. May 27, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html. Accessed June 14, 2021.
5. Park JW, Lagniton PNP, Liu Y, Yu RH. MRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17(6):1446-1460.
6. Katella K. Comparing the COVID-19 vaccines: how are they different? Yale Medicine. Updated August 13, 2021. www.yalemedicine.org/news/covid-19-vaccine-comparison. Accessed August 23, 2021.
7. Yocum A, Simon EL. Thrombotic thrombocytopenia purpura after Ad26.COV2-S vaccination. Am J Emerg Med. May 4, 2021: S0735-6757(21)00376-4. https://pubmed.ncbi.nlm.nih.gov/33980419/. Epub ahead of print. Accessed June 14, 2021.
8. CDC. COVID-19 Safety & monitoring. Updated July 13, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html. Accessed August 23, 2021.
9. Ciccone A. SARS-CoV-2 vaccine-induced cerebral venous thrombosis. Eur J Intern Med. 2021;89:19-21.
10. Parums DV. Editorial: SARS-CoV-2 mRNA vaccines and the possible mechanism of vaccine-induced thrombotic thrombocytopenia (VITT). Med Sci Monit. 2021;27:e932899.
11. Mehta PJ, Mangion SA, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—a report of two UK cases. Brain Behav Immun. 2021;95:514-517.
12. Elelamy I, Gerotziafas G, Alamowitch S, et al. SARS-CoV-2 vaccine and thrombosis: expert consensus on vaccine-induced thrombosytopenia. Thromb Haemost. 2021;121(08):982-991.
13. World Health Organization. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19): interim guidance. July 19, 2021. www.who.int/publications/i/item/WHO-2019-nCoV-TTS-2021.1 Accessed August 5, 2021.
14. Porres-Aguilar M, Lazo-Langner A, Panduro A, Uribe U. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: an emerging cause of splanchnic vein thrombosis. Ann Hepatol. 2021;23:100356.
15. Gomez-Mesa JE, Galindo-Coral S, Montes MC, et al. Thrombosis and coagulopathy in COVID-19. Curr Probl Cardiol. 2021;46(3):100742.
16. See I, Su JR, Lale A, Munoz Martin AJ. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-2456.
To comment on this article, contact rdavidson@uspharmacist.com.






