US Pharm. 2023;48(12):44-47.
ABSTRACT: Dengue virus (DENV) is an infectious disease that is transmitted by Aedes mosquitoes. The widespread population of these mosquitoes has contributed to an important public health issue, as this mosquito-borne virus is endemic in many areas, especially in tropical and subtropical regions, and developing dengue can lead to severe symptoms that can be fatal. Dengue infections can be asymptomatic, mild-to-moderate, or severe, depending on the individual. Dengvaxia is approved for vaccination in at-risk children who have been previously infected with dengue. Pharmacists can advocate for vaccination, provide education about safe travel practices, and assist in managing patients with dengue.
Dengue, which is caused by the dengue virus (DENV), is a mosquito-borne infectious disease that has contributed to multiple outbreaks and epidemics within the populations of over 100 countries. Tropical and subtropical regions are typically more prone to dengue outbreaks, but infections have been reported worldwide. Due to the widespread prevalence of Aedes species mosquitoes, the causative vector for transmitting DENV, dengue has become an important public health issue, as severe dengue infections can be fatal. Knowledge of clinical presentation and management is an important aspect in limiting the severity of dengue symptom manifestation in patients. Healthcare providers, including pharmacists, can contribute to this pursuit of achieving improved patient outcomes in those infected with DENV.1
Epidemiology and Etiology
Nearly 4 billion people are at risk of developing dengue, which may be asymptomatic or symptomatic. While approximately 400 million people worldwide are infected with DENV each year, an estimated 100 million people become sick from DENV. Of those individuals, 40,000 die annually from a severe manifestation of dengue.2 The World Health Organization (WHO) reports that in 2023 there have already been approximately 3 million confirmed cases of DENV in the regions of North and South America, exceeding the 2.8 million total confirmed cases from 2022.1 The CDC reports that from 2010 to 2020, people aged 19 years and younger were more likely to become infected with DENV; however, since 2021, cases in adults aged older than 50 years have increased, while cases in children and young adults have declined.2 The increased incidence of dengue and selected outbreaks in the United States reflect the increase in international travel.1,2 An estimated 1,300 cases of dengue have occurred in the U.S. as of October 2023.2
Dengue infections are caused by one of following four DENV serotypes: DENV 1, DENV 2, DENV 3, or DENV 4.1,2 When a person becomes infected with one serotype of DENV, that person does not become fully immune to the other three serotypes; therefore, a person can still become infected with the other three serotypes of DENV upon exposure. Since DENV is transmitted by mosquito bites and/or contact with bodily fluids, people who have traveled to another country can become infected and then travel home, spreading dengue. Additionally, the widespread population of Aedes mosquitoes (Ae. aegypti or Ae. albopictus) increases the risk of transmissibility. Proper management of water, public health initiatives, and other sanitation measures can help limit mosquito populations to an extent, but dengue is still endemic in many areas.1-5
Clinical Presentation and Diagnostic Criteria
The manifestation of dengue symptoms varies among infected individuals; some people are asymptomatic, while others are severely ill. Some people develop mild-to-moderate symptoms, such as headache, high fever, nausea, emesis, myalgia, arthralgia, etc. Once a person becomes infected with DENV, however, that person is at risk for developing severe dengue upon another exposure. Severe dengue can be life-threatening and even fatal due to the risk of severe bleeding and hypovolemic shock; dengue shock syndrome (DSS) is a severe form of dengue. Severe dengue is characterized by the aforementioned symptoms, as well as tachypnea, hemorrhaging, weakness, dehydration, and shock.6,7 Infants are at a higher risk for DSS due to a reduced ability to adapt to the hemorrhaging that can occur with DENV.7
Prior to 2009, the WHO categorized dengue into one of the following diagnoses: dengue fever (DF), dengue hemorrhagic fever (DHF), and DSS.7 However, the 2009 WHO guidelines include an updated categorization method for dengue that differentiates between severe dengue and nonsevere dengue.7 The diagnostic criteria and management of severe dengue (DSS) and nonsevere dengue, including probable dengue, dengue without warning signs (DF), and dengue with warning signs (DHF), are listed in TABLE 1.
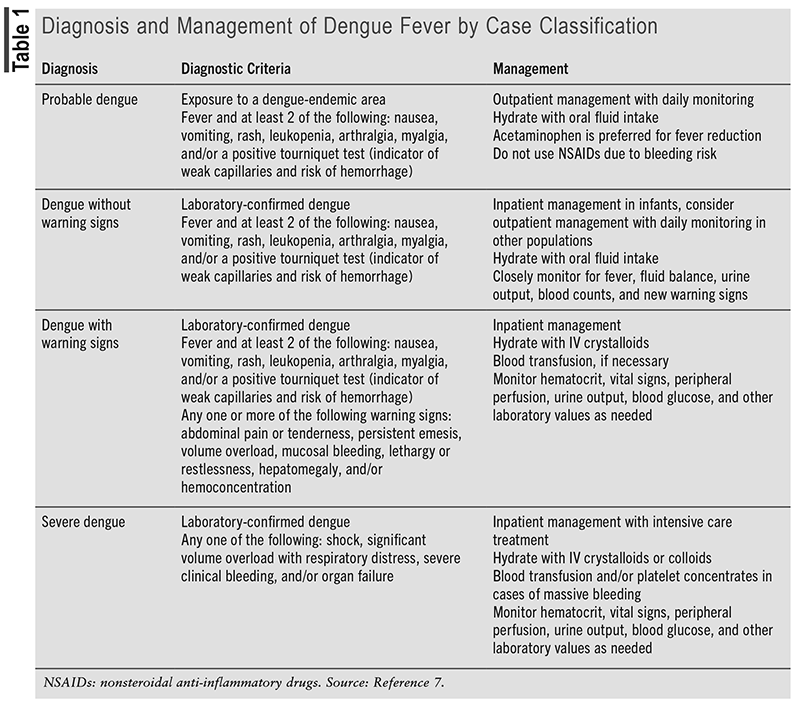
Dengue is typically a self-limiting disease, with an incubation period of around 4 to 7 days. Viremia usually occurs during a febrile episode. The most common laboratory abnormalities include leukopenia, thrombocytopenia, and liver enzyme elevation. Severe dengue is characterized by an abrupt onset of hemorrhagic manifestations, with or without shock, during fever-free periods.7
Disease Prevention and Management
In adults and dengue-naïve children, the most effective method for infection prevention is by protecting oneself from mosquito bites while traveling to endemic countries. This can be done by using insect repellents and wearing long-sleeved shirts and pants while in these endemic areas.2 There is no treatment for dengue infection; however, a live vaccine, Dengvaxia, has recently been developed and approved by the FDA to help prevent dengue in children aged 6 through 16 years who have a laboratory-confirmed previous dengue infection and who live in a dengue-endemic area. Laboratory confirmation can be obtained by the following: evidence of prior acute dengue infection via a positive dengue reverse transcriptase–polymerase chain reaction (RT-PCR) result or a positive dengue nonstructural protein 1 (NS1) antigen test result.7-9 Confirmation of a previous dengue infection before vaccine administration is necessary because there is an increased risk of viremia in patients who have no prior history of dengue. Dengvaxia, also referred to as chimeric yellow fever virus dengue-tetravalent dengue vaccine (CYD-TDV), provokes dengue-specific immune responses against the four DENV serotypes after administration.9,10 The safety and efficacy of Dengvaxia have been confirmed in multiple randomized clinical trials.10-12
Capeding et al conducted a phase III, randomized, multicenter, observer-masked trial in Asia that evaluated the safety and clinical efficacy of the dengue vaccine in children aged 2 to 14 years.10 The primary end point was to determine the efficacy of the vaccine against symptomatic and virologically confirmed dengue infection at 28 days after completion of the three-dose vaccine series. The study achieved the primary end point with 56.5% efficacy (95% CI, 43.8-66.4).10 One serious adverse event, determined to be vaccine-related, occurred in a participant who developed acute disseminated encephalomyelitis on Day 7 after receiving the first injection, but it resolved within 15 days and did not recur.10
Sridhar et al performed a follow-up, case-cohort, retrospective study that confirmed the impact theory of serostatus of the individuals, based on blood taken at 13 months, post vaccination.11 The study found that there was an increased risk of hospitalization and severe dengue in seronegative individuals.11
Another phase III, multicenter, randomized, double-blinded trial was conducted by Villar et al in Brazil, Colombia, Honduras, Mexico, and Puerto Rico to determine dengue vaccine efficacy in children aged 9 to 16 years.12 The primary end point was the efficacy of the dengue vaccine against symptomatic and virologically confirmed dengue infection. The vaccine was demonstrated to be 60.85% effective (95% CI, 52.0-68.0) in the per-protocol population, and in the intention-to-treat group participants who received at least one injection, there was a vaccine efficacy of 64.7% (95% CI, 58.7-69.8).12 Total vaccine efficacy against hospitalizations for virologically confirmed dengue after at least one dose of vaccine was 80.3%. The secondary end points focused on the efficacy of the dengue vaccine in symptomatic prevention, hospitalization occurrence, antibody response to each dengue serotype, and the occurrence of serious adverse events in the participants throughout the trial period.12 Adverse events that were found to be vaccine-related included an asthma attack 16 hours after the first injection, allergic urticaria 4 hours after the second injection, and acute peripheral polyneuropathy related to viral meningitis 3 days after the first injection.12,13
For those who become infected with dengue, there is no specific treatment, so supportive care based on symptoms and symptom severity is the mainstay of therapy.7 Patients with confirmed dengue infection should be assessed for the need for either outpatient or inpatient treatment. Outpatient treatment is sufficient for most otherwise healthy infected individuals who are able to tolerate fluids and who have normal blood counts. Preexisting conditions that may warrant inpatient therapy include diabetes, kidney disease, obesity, or people within special populations (i.e., pregnant, elderly, infantile).7 Acetaminophen is preferred for fever reduction, as nonsteroidal anti-inflammatory drugs can further bleeding complications associated with dengue infection. Patients should also receive fluid resuscitation, as needed, based on symptoms and clinical status. Complications of dengue infection, including but not limited to plasma leaking, shock, bleeding, and altered mental status, should be addressed and managed on an individualized basis, as described in TABLE 1.7
Discussion
Nearly 4 billion people reside in areas with the DENV, and with international travel to endemic regions becoming common again post the COVID-19 pandemic, there is a heightened risk of contracting the virus.1,2 Due to the recent uptick of international travel, domestic outbreaks of dengue in the U.S. have not been spared. As of early August 2023, there have been reported dengue outbreaks in South Florida.14 Most cases have been travel-associated infections in individuals returning from endemic regions. By the end of August 2023, there were 244 dengue cases reported in people who had traveled to an area with dengue within 2 weeks prior to onset. Four of the cases were classified as severe dengue, and 16 of the cases were classified as cases acquired in Florida in 2023.14
Dengvaxia is the first vaccine indicated for prevention of dengue in children aged 6 to 16 years to become available in the U.S.9,10 Previously, prophylaxes consisting of the use of physical barriers, such as long-sleeved clothing and application of insect repellant, were the only methods of infection prevention. Other methods used to control or prevent the transmission of the DENV include the proper disposal of solid waste and the removal of artificial, man-made Aedes habitats.6,7 Active monitoring and surveillance of vectors play a vital role in dengue infection prevention.15 Reasons for continual transmission include, but are not limited to, environmental change, population growth, rapid urbanization, and inadequate water storage.2,3,6,7
Role of the Pharmacist
As one of the most accessible healthcare professionals, the pharmacist plays a vital role in providing education about safe travel and preventing infections through advocating for appropriate immunizations in those who live in dengue-endemic areas. Pharmacists are positioned to assist patients with medication recommendations and prevention of travel-related diseases. Pharmacists can assist patients with selecting OTC medications and other necessary prophylactic medications for travel; additionally, they can administer immunizations and dispense travel medications. Patients have recognized the accessibility of vaccination by community pharmacists, which can help to increase vaccine awareness and potentially prevent the transmission of infections such as dengue. As a result, pharmacists are ideal healthcare professionals with whom to establish collaborative practice agreements, which can help reach more patients requiring travel medicine and also potentially reduce the burden of communicable diseases worldwide.16
Conclusion
The endemic classification of dengue presents a public health issue that can cause life-threatening, even fatal, effects in people who become infected. Now that international travel has increased, prevention methods are necessary to promote on a worldwide scale. With the recent development and FDA approval of Dengvaxia for children and adolescents aged 6 to 16 years who have experienced dengue infection and who live in areas where the disease is commonly contracted, a vaccine is available for prevention. This vaccine provides coverage against all four serotypes, after only three injections, over a 12-month period.9 Although Dengvaxia has demonstrated efficacy and safety, all individuals should still wear protective clothing and insect repellent when traveling to or living in areas where dengue is most prevalent.2,6,7 If dengue is contracted, patients should be assessed for inpatient or outpatient treatment based on symptoms and risk factors and treated with supportive care.7
Pharmacists can play a vital role in both preventing and managing dengue by advocating for safe travel and vaccination, when appropriate, and by assisting the medical team with appropriate supportive care.
REFERENCES
1. World Health Organization. Disease Outbreak News. Dengue in the Region of the Americas. www.who.int/emergencies/disease-outbreak-news/item/2023-DON475. Accessed September 17, 2023.
2. CDC. Dengue. www.cdc.gov/dengue/index.html. Updated August 15, 2023. Accessed September 17, 2023.
3. World Health Organization. Vector-borne diseases. www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/. March 2, 2020. Accessed September 10, 2023.
4. Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564-581.
5. Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100-103.
6. World Health Organization. Dengue and severe dengue. www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. March 17, 2023. Accessed September 15, 2023.
7. World Health Organization. Dengue guidelines for diagnosis, treatment, prevention, and control. 2009. Accessed September 20, 2023. https://apps.who.int/iris/bitstream/handle/10665/44188/9789241547871_eng.pdf?sequence=1.
8. CenterWatch. Dengvaxia (Dengue Tetravalent Vaccine, Live). www.centerwatch.com/drug-information/fda-approved-drugs/drug/100382/dengvaxia-dengue-tetravalent-vaccine-live-. Accessed September 10, 2023.
9. Dengvaxia (package insert). Swiftwater, PA: Sanofi Pasteur, Inc. www.fda.gov/media/124379/download?attachment. 2023.
10. Capeding MR, Tran NH, Hadinegoro SR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358-1365.
11. Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327-340.
12. Villar L, Dayan GH, Arredondo-García JL, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372(2):113-123.
13. Cunaha J. Dengvaxia. RxList. www.rxlist.com/dengvaxia-side-effects-drug-center.htm. Accessed September 16, 2023.
14. Florida Department of Health. Mosquito-borne disease surveillance. www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/surveillance.html. Accessed September 17, 2023.
15. Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760.
16. Le LM, Veettil SK, Donaldson D, et al. The impact of pharmacist involvement on immunization uptake and other outcomes: an updated systematic review and meta-analysis. J Am Pharm Assoc. 2022;62(5):1499-1513.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





