US Pharm. 2012;37(11):29-32.
ABSTRACT: Depression is more prevalent in patients with epilepsy than in the general population. The condition remains underdiagnosed because of underreporting of signs and symptoms. Another reason for lack of treatment is the belief that antidepressants have proconvulsant effects. Many antidepressants are known to lower the seizure threshold; however, data indicate that, at low doses, antidepressants possess anticonvulsant properties. Evidence also suggests that when an antidepressant is used within its therapeutic dosage range, the risk of seizure activity is low. When selecting an antidepressant for use in a patient with epilepsy, the clinician should carefully consider drug-drug interactions between antiepileptics and antidepressants. In general, selective serotonin reuptake inhibitors are considered first-line therapy. The efficacy of antidepressants in epilepsy patients may be enhanced with supportive therapy or psychotherapy.
The prevalence of depression is significantly higher in epilepsy patients than in the general population. Depression is the most common comorbid disorder in patients suffering from epilepsy, and it is more frequent and severe in epilepsy patients than in patients with chronic disorders or neurologic conditions. The incidence and prevalence of depression in the epilepsy population are difficult to establish, mainly because of the underreporting and underdiagnosis of depressive symptoms. Additionally, the diverse methodologies and sample populations used across studies yield drastically different study conclusions.1,2
Compared with the general population, the rate of depression in patients with recurrent seizures is 20% to 55%.1 In patients with controlled epilepsy, the rate of depression is 3% to 9%.1 Community-based studies of epilepsy populations report depression rates of 9% to 22%, and hospital-based samples report much higher rates (27%-58%).2 Health-related quality of life is worse in patients with recurrent seizures compared with those who had no seizure episodes in the last several years.3 Compared with patients who did not have seizures in the previous year, patients with recurrent seizures are five times more likely to experience depression.3 Untreated depression can lead to an increased risk of suicide, which is one of the foremost causes of death in epilepsy patients.4 Suicide in patients with epilepsy is estimated to be about 10 times higher than in the general population.1
Pathology and Diagnosis
Epilepsy is defined as the occurrence of two unprovoked seizures separated by 24 hours.5 A seizure results from a disturbance of electrical activity in the brain that leads to changes in attention and/or patient behavior.5,6 In epilepsy, permanent alterations in brain tissue cause hyperexcitation of the brain, which in turn emits abnormal signals that can lead to unpredictable seizures.5
As previously noted, the most common comorbid disorder in epilepsy is depression.1 The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision), defines major depressive disorder (MDD) as one or more major depressive episodes (≥2 weeks of depressed mood or loss of interest accompanied by ≥4 additional symptoms of depression, e.g., weight/appetite changes, sleep disturbances, psychomotor symptoms, fatigue, feelings of worthlessness, executive-function deficit, suicidal ideation).7 Symptoms must cause significant impairment in social, occupational, or other important areas of functioning, and they cannot be attributed to psychological effects of a substance or to bereavement.
Changes in serotonin and noradrenaline play a key role in the pathology of MDD and epilepsy.1 Although the exact pathology is unknown, MDD involves catecholamines (dopamine, noradrenaline, and serotonin) and neurotransmitters (glutamate and gamma-aminobutyric acid [GABA]).8 Similarly, in epilepsy, temporal imbalances between the main neurotransmitters (e.g., glutamate) and the catecholamines (e.g., serotonin) increase the risk of seizures in susceptible patients.5 Furthermore, reduced catecholamine functioning (serotonergic and noradrenergic) has been shown to worsen seizure severity in some animal models.1
Depressive symptoms should be classified according to their occurrence in relation to seizure onset.4 In preictal depression, the depressed mood occurs hours to days prior to a seizure episode and is relieved by the onset of convulsions.9 In ictal depression, which is often described as an aura signifying seizure onset, the depressed mood occurs minutes before onset of a complex partial seizure.4 Some patients view preictal and ictal depression as a warning sign of seizure that allows them to inform others and relocate to a safe place.9 Postictal depression is characterized by a depressed mood that develops hours to days following a seizure episode. Interictal depression tends to present as chronic depression in epilepsy patients.4
Risk of Seizure With Antidepressants
Many antidepressants are known to lower the seizure threshold, which in turn may provoke seizures in patients, especially those who already have predisposing risk factors for seizures. The mechanism by which antidepressants cause seizures is not fully understood.10 Initially it was thought that because antidepressants inhibit serotonin and/or norepinephrine reuptake, the convulsant properties were secondary to the antidepressant effects. This has since been shown to be unlikely and, in fact, accounts for the anticonvulsant properties of antidepressants at lower doses. For example, in experimental settings, amitriptyline has been shown to reduce spike activity at lower concentrations.11,12 Other proposed mechanisms for the convulsant properties of antidepressants include effects on glutamatergic, GABAergic, and histaminergic neurotransmission, G protein–coupled K+ channels, and brain-derived neurotrophic factor. Data are insufficient to determine whether these proposed mechanisms are the definitive cause of the convulsant properties of antidepressants.11
The first antidepressant reported to cause seizures in patients treated for depressive illness was imipramine, a tricyclic antidepressant (TCA).11 At therapeutic doses, the TCAs’ seizure rate ranges from 0.4% to 2%. In general, the risk should be considered similar in each of the TCAs.10 The tetracyclic antidepressants (TeCAs) maprotiline and amoxapine have been associated with a higher rate of seizures. Postmarketing data indicated that maprotiline had a strong dose-effect relationship, and as a result the therapeutic dosage range was lowered (maximum 225 mg/day). Monoamine oxidase inhibitors are believed to have a relatively low risk of seizures. Trazodone is considered to have a low risk of seizures, although seizures have been reported with its use.10 Newer-generation antidepressants are considered safe and more tolerable. The incidence of seizures for newer-generation antidepressants is lower than that for TCAs and TeCAs (0%-0.4%).10 The risks of seizures with the newer antidepressants have been examined in several reviews and research articles (TABLE 1).10,13,14 Factors that affect the range of risk are dose and predisposing factors.10
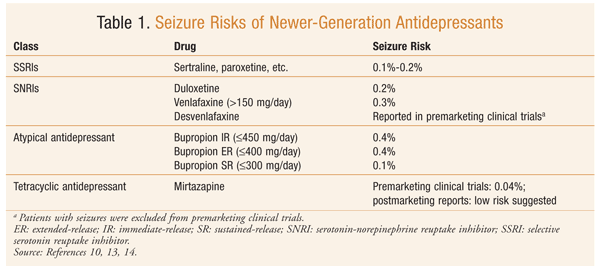
A review assessed the number of reported convulsions associated with antidepressants and other neuroleptic agents in VigiBase, the World Health Organization’s adverse drug reaction (ADR) database, between 1986 and 2006.15 The percentage of reports for antidepressants and convulsions ranged from 1.23% to 14.43%; the highest amount was reported for maprotiline (14.43%), followed by escitalopram (9.78%), bupropion (9.48%), amoxapine (8.74%), trimipramine (5.69%), and clomipramine (5.6%).15 A limitation of the VigiBase data is that a causal relationship between the drug and the reported ADR is not established; instead, the report serves as a means of early detection of ADRs.15
An analysis of FDA Summary Basis of Approval (SBA) reports for all antidepressants approved between 1985 and 2004 was conducted.16 (The SBA report is a review of preclinical and clinical data from a drug’s New Drug Application.) Bupropion IR had the highest incidence of seizure (0.6%), followed by citalopram (0.3%); differences between the other antidepressants were not significant.16
Pharmacologic Treatment
In treating a patient with epilepsy and depression, the first priority should be to achieve optimal control of seizures using appropriate anticonvulsant therapy.2 Some anticonvulsants, such as valproate, carbamazepine, lamotrigine, and gabapentin, have demonstrated mood improvement in patients with epilepsy. They have also shown efficacy in preventing manic and depressive episodes in bipolar patients.2 These drugs may thus be beneficial to epilepsy patients suffering from depression.
Prior to initiating treatment of depression in a patient with epilepsy, it is important to ascertain that the depressive episodes are not caused by changes to the antiepileptic drug regimen. For example, discontinuing carbamazepine, valproic acid, or lamotrigine—all of which have mood-stabilizing properties—can lead to depressive episodes.1 In such cases, reintroducing the antiepileptic or initiating a mood-stabilizing agent may be sufficient to achieve a euthymic state.1 Another example would be a patient experiencing a depressive episode following the introduction or incremental dosing of an antiepileptic with known negative psychotropic properties. Lowering the dosage or discontinuing the drug, if feasible, will lead to symptom remission.1 However, if a patient is taking an antiepileptic that has negative psychotropic properties (e.g., phenobarbital, primidone, tiagabine, topiramate, vigabatrin) but is known to confer superior seizure control, the depressive episode may be treated with a selective serotonin reuptake inhibitor (SSRI), such as sertraline or paroxetine.4
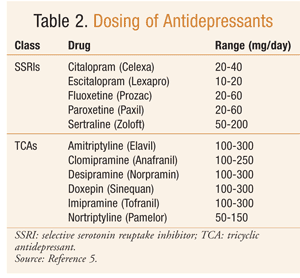
The drug of choice for treating depression in a patient with epilepsy depends upon the most prominent depressive symptoms the patient exhibits, as well as the drug’s efficacy, interaction with antiepileptics, and ADR profile.9 Effective antidepressive treatment may indirectly improve seizure control owing to the fact that adequately treated patients have improved sleep patterns and are more adherent to their antiepileptic drug regimen.17 In general, SSRIs are considered first-line treatment in patients experiencing depression and seizures (TABLE 2).2 SSRIs are unlikely to exacerbate seizure onset, are less likely to result in death following an overdose, and have a good ADR profile.4 TCAs also have shown good clinical response in the treatment of depression in epilepsy patients (TABLE 2).9 TCAs have a low risk of exacerbating seizures when used within therapeutic range.2 However, due to potential cardiotoxic effects and severe complications occurring with overdose, TCAs are used second line.1 To lessen the chance of relapse, therapy should be continued for 4 months after the last symptoms of depression abate.9
Nonpharmacologic Treatment
Depressive reactions can be treated with supportive therapy, counseling, and rehabilitation. Supportive therapy should be offered to patients newly diagnosed with epilepsy, as well as to their family members.9 Supportive therapy will help educate patients and their family members about epilepsy and determine a patient’s state of mind regarding, and emotional reaction to, the disorder. In addition, supportive therapy can help eliminate misinformation about epilepsy and teach patients and their families how to cope.9 More severe reactions may require psychotherapy (e.g., cognitive-behavioral therapy), which can improve a patient’s coping skills.9 In some cases in which a patient does not respond to antidepressants, or in a situation in which antidepressant use increases the patient’s risk of seizures, electroconvulsive therapy (ECT) may be used as an alternative treatment. ECT has been shown to be well tolerated among epilepsy patients.4
Drug-Drug Interactions
Most antidepressants inhibit one or more CYP450 isoenzymes and are metabolized in the liver.2 SSRIs’ inhibition of P450 can result in toxic levels of antiepileptics such as phenytoin, phenobarbital, and carbamazepine. Elevated levels carbamazepine levels were observed when the drug was given with fluoxetine, fluvoxamine, or nefazodone.2 Coadministration of fluoxetine and carbamazepine can lead to toxic serotonin syndrome, which is characterized by uncontrollable shivering, agitation, incoordination, restlessness in the feet when sitting, involuntary contractions progressing to myoclonic-like leg movements, and hyperreflexia.9 The SSRI citalopram has no pharmacokinetic interactions with antiepileptic drugs and thus may be used as an alternative.4
Antiepileptic agents such as phenytoin, carbamazepine, phenobarbital, and primidone are potent CYP450 enzyme inducers.2 A result of this inducing effect is the accelerated metabolism of antidepressants.4 This accelerated metabolism occurs especially in the TCA class and in paroxetine.2,9 However, the inducing effect is significantly reduced with the newer antiepileptics (e.g., gabapentin, lamotrigine, levetiracetam).4
Some antidepressants (e.g., SSRIs) and antiepileptics (e.g., barbiturates, benzodiazepine) cause sedation and cognitive impairment.2 This may lead to daytime drowsiness and/or impaired psychomotor functioning in the patient.9
Conclusion
Depression is a common comorbidity in patients with epilepsy.1 Although the mechanism is not fully understood, antidepressants are associated with lowering the seizure threshold, which may induce or provoke seizure, particularly in those with a predisposed risk. As a result, prescribers may be hesitant to use antidepressants in epilepsy patients. Further research has shown that, at lower doses, antidepressants possess anticonvulsant properties and that, at therapeutic doses, the risk of seizure activity is minimal. It is suggested that patients with epilepsy and depression be treated with psychotherapy; if medication is indicated, SSRIs are generally considered first line because of their safety, tolerability, and ADR profile. ECT is a viable treatment option for patients with severe depression and epilepsy who are unable to take antidepressants. In selecting an antidepressant to be used in a patient with epilepsy, consideration should be made with regard to drug-drug interactions with the antiepileptic drugs, the potential for seizure activity, and the prominent depressive symptoms exhibited by the patient.
REFERENCES
1. Kanner A. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388-398.
2. Harden C. The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology. 2002;59(6 suppl 4):S48-S55.
3. Cramer JA, Blum D, Reed M, et al. The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav. 2003;4:515-521.
4. Gilliam F, Kanner A. Treatment of depressive disorders in epilepsy patients. Epilepsy Behav. 2002;3(5 suppl):2-9.
5. Rogers SJ, Cavazos JE. Epilepsy. In: Talbert RL, DiPiro JT, Matzke GR, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill Medical; 2011.
6. Epilepsy. PubMed Health. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001714. Accessed July 28, 2012.
7. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision). Arlington, VA: American Psychiatric Association; 2000.
8. Dell’Osso B, Palazzo MC, Oldani L, Altamura AC. The noradrenergic
action in antidepressant treatments: pharmacological and clinical
aspects. CNS Neurosci Ther. 2011;17:723-732.
9. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia. 1999;40(suppl 10):S21-S47.
10. Montgomery SA. Antidepressants and seizures: emphasis on newer agents and clinical implications. Int J Clin Pract. 2005;59:1435-1440.
11. Jobe PC, Browning RA. The serotonergic and noradrenergic effects
of antidepressant drugs are anticonvulsant, not proconvulsant. Epilepsy Behav. 2005;7:602-619.
12. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91-110.
13. Pristiq (desvenlafaxine) product information. Philadelphia, PA: Wyeth Pharmaceuticals Inc; October 2011.
14. Khawam EA, Laurencic G, Malone DA Jr. Side effects of antidepressants: an overview. Cleve Clin J Med. 2006;73:351-353,356-361.
15. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19:69-73.
16. Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in
psychopharmacological clinical trials: an analysis of Food and Drug
Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62:345-354.
17. Thomé-Souza MS, Kuczynski E, Valente K. Sertraline and
fluoxetine: safe treatments for children and adolescents with epilepsy
and depression. Epilepsy Behav. 2007;10:417-425.
To comment on this article, contact rdavidson@uspharmacist.com.






