US Pharm. 2010;35(1)(Oncology suppl):4-12.
ABSTRACT: The number of hematologic agents available is constantly growing. As a result, health care practitioners often encounter medications they are unfamiliar with, which may contribute to an increased potential for patient harm and/or inadequate response, unnecessary product waste, and confusion among clinicians. The use of hematologic agents requires careful consideration of the variables surrounding their utilization. Several issues are paramount in the optimization of pharmacotherapy, including the impact of patient comorbidities on treatment, appropriately preparing and administering medications, and product acquisition. This becomes increasingly important with hematologic agents, as these drugs often have narrow therapeutic indices, involve more complex dosing regimens, and are more costly. Three patient cases and agents are reviewed: 1) intravenous immunoglobulin (IVIG) in the management of idiopathic thrombocytopenic purpura; 2) hemin in the treatment of acute intermittent porphyria; and 3) azacitidine in the treatment of myelodysplastic syndrome. These cases illustrate a step-wise approach used in the selection of the optimal treatments given specific circumstances. The approach used in each of these cases may be applied to various patient care settings in an effort to improve medication utilization and outcomes.
With the growing number of hematologic agents available, it is critical for health care practitioners to become familiar with practical handling considerations to ensure safe and effective use of these products.1 Many medications that fall within the hematologic agent category are administered via the IV route and, therefore, are at a particularly high risk for errors.2 Additionally, this class of drugs is often associated with a narrow therapeutic index. The risk of patient harm, inadequate response, and product waste can be avoided or minimized by developing and implementing procedures that provide guidance on admixture and administration and appropriate selection of treatments. Using an approach to handle a patient case affords the practitioner the ability to recognize and account for treatment-dictating variables.
To highlight the use of a step-wise approach in treatment selection, three patient cases are shared and discussed: 1) intravenous immunoglobulin (IVIG) (various manufacturers) in the management of idiopathic thrombocytopenic purpura (ITP); 2) hemin (Panhematin) in the treatment of acute intermittent porphyria (AIP); and 3) azacitidine (Vidaza) for myelodysplastic syndrome (MDS). These agents were selected for discussion because their use is relatively infrequent and involves several caveats many practitioners may be unfamiliar with. This is a common trait among hematologic agents.
Each case will review the medication process from treatment selection through administration and identify practical strategies to address issues identified during this process. The step-wise process used in each of the case discussions may be applied to various situations.
CASE 1: IVIG in ITP
MN is a 49-year-old African-American female diagnosed with chronic ITP 3 years ago. Her current presentation is significant for thrombocytopenia (platelets 19,000/mm3), petechiae, known history of chronic ITP, hypotension (90s/60s), and hemoptysis. Significant laboratory values include hemoglobin 6.8 mg/dL, platelets 19,000/mm3, and a serum creatinine of 1.7 mg/dL. Her past medical history is significant for peptic ulcer disease (PUD), deep venous thrombosis secondary to hormone therapy, obesity (BMI 43 kg/m2), and diabetes mellitus.
MN’s diagnosis of ITP is consistent with the common findings in classic chronic ITP: thrombocytopenia, female gender (female:male; 2.1:1), age between 20 and 50 years, nonpalpable petechiae, and bleeding.3,4 Treatment is generally unnecessary in adults when platelet counts are greater than 30,000/mm3 in the absence of bleeding or comorbidities that confer an increased risk of bleeding, such as uncontrolled hypertension, recent surgery, active PUD, or head trauma.5,6 Corticosteroids and IVIG are generally considered first-line emergency treatment.7 When deciding whether to administer corticosteroids, IVIG, or a combination of both during an acute ITP exacerbation, careful consideration of patient comorbidities aids in treatment selection. If a more rapid increase in platelets is required due to symptoms or situations where bleeding is imminent (e.g., surgical procedure, pregnancy), IVIG may be preferred due to a more rapid platelet response.7 With a platelet count of 19,000/mm3, typical symptoms of ITP, bleeding, and a history of ITP, pharmacotherapy is warranted in MN.
A diagnosis of chronic ITP exacerbation is made and therapy with corticosteroids and IVIG is initiated. The dosage of IVIG administered (1 g/kg × 2 doses) is consistent with the product labeling (Gammagard S/D) of the IVIG product on formulary.8 Currently, there are four FDA-approved indications for IVIG (Gammagard)—ITP, Kawasaki syndrome, primary immunodeficiency diseases, and B-cell chronic lymphocytic leukemia; however, IVIG is often used off-label.9 A recent evaluation identified 150 off-label indications for IVIG in the literature.9 Although there may be off-label indications with strong data, many do not have sufficient evidence to warrant use. A Pharmacy and Therapeutics Committee–approved IVIG indication policy will help restrict IVIG usage to the most appropriate indications.
Several considerations are critical to evaluate prior to initiating IVIG, including risk of bleeding, thrombosis, and renal failure. The patient’s past medical history is significant for thrombosis and PUD. Given the patient’s history of PUD in the setting of thrombocytopenia, prompt management is of utmost importance due to the potential of worsening bleeding. Delays in treatment must be avoided to prevent further complications from arising.
Practical issues that assist in preventing delays include appropriate admixture, prompt delivery to the patient, and appropriate administration technique. TABLE 1 summarizes key strategies to address potential concerns with IVIG. Due to the difficult dissolution of IVIG, inappropriate admixture technique has the potential to delay medication delivery, thus impacting practitioner and patient perception of the institution’s medication processes. Health care professionals should avoid vigorously shaking the drug during the reconstitution process due to the likelihood of foaming. Gentle swirling or the use of a reciprocating shaker may expedite drug dissolution. Sterile water for reconstitution should be allowed to warm to room temperature to speed dissolution. Certain preparations may require the release of the vacuum to allow complete dissolution. The availability of newer liquid formulations of IVIG that do not require reconstitution may minimize the potential delays arising from admixture technique. Due to the number of IVIG products available and the nuances of each product, practitioners should refer to the prescribing information for specific information on admixture. IVIG administration should occur shortly after admixture (within 2 hours); therefore, prompt delivery is crucial. Another important issue with IVIG and other IV medications is recognizing what the reconstituted product should look like (IVIG has a transparent gel-like appearance). If one is visually inspecting a product he or she is unfamiliar with, problems with the product may pass undetected.
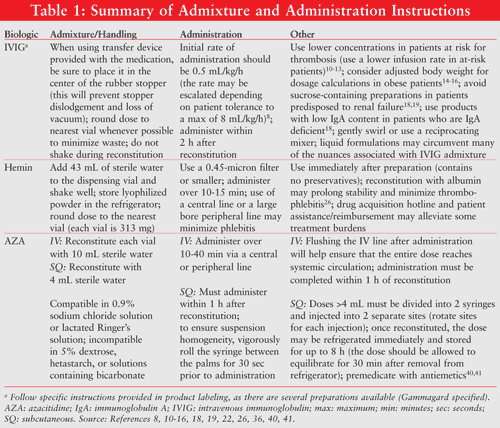
MN’s history of thrombosis is also of concern since IVIG may increase blood viscosity, thus further increasing risk of thrombosis.10-12 Other potential mechanisms by which IVIG may increase the risk of thrombosis include IVIG-induced platelet aggregation, arterial vasospasm, alterations in cytokine profile, and contamination of IVIG products with activated coagulation factor XI.11 There are several administration-related strategies that practitioners may utilize to reduce the risk of thrombosis. First, use lower concentrations and lower rates of infusion (0.5 mL/kg/h).12 This strategy may be used for all IVIG products, since thrombotic complications have been reported with various formulations. Although there are conflicting data in the literature, the FDA has suggested that high doses and high infusion rates of IVIG may increase the risk of thrombosis.13 It has been suggested that using the ideal body weight for dosage calculations may decrease adverse events.14,15 However, this has not been specifically studied. The use of an adjusted body weight in obese patients may also help minimize thromboembolic complications. Dosing is usually based on actual body weight, which in the obese population may result in a high intravascular concentration, predisposing patients to hypercoagulability due to increased serum viscosity.10 Practitioners should also avoid concomitant administration of blood products, as they too may increase blood viscosity, thus further increasing the risk of a thrombotic event.16 Finally, the use of prophylactic aspirin for patients at high thrombotic risk may be considered as part of a protocol to minimize risk of thrombotic complications.17 Unfortunately, aspirin therapy may not be applicable in the case of MN due to the presence of active bleeding.
In addition to thrombosis, acute renal failure has also been reported with IVIG. This is a concern given the patient’s renal status. This adverse effect is likely related to the use of sucrose as a stabilizer in certain IVIG formulations and preparations with high osmolarity.18,19 Certain conditions may increase the risk of renal failure, such as concomitant nephrotoxic medications, diminished circulation, acute infection, or advanced age. Selection of a formulation utilizing an alternative stabilizing agent to sucrose may be appropriate in patients with poor renal status. The sucrose-containing IVIG formulations are Carimune NF, CytoGam, Sandoglobulin, Panglobulin, and Gammar-P IV/Gammar-IV.19 The only sugar-free IVIG formulations are Gammagard Liquid 10%, Gamunex, and Vivaglobin. Although the majority of acute renal failure reports were in patients treated with sucrose-stabilized preparations, it may be prudent to use lower concentrations or infusion rates to avoid this complication in all currently available preparations.
Other potential adverse effects of IVIG include hypotension, aseptic meningitis, and anaphylaxis. Since MN already has hypotension, care should be taken to ensure she is not immunoglobulin A (IgA) deficient. The risk of anaphylaxis has been associated with higher IgA content in the presence of IgA deficient patients.18 Use of a specific IVIG product with the lowest IgA content, such as Gammagard S/D, may help minimize this complication in these patients. As with any product derived from human donation, the potential for transmissible infection, although extremely rare, should be considered.
In summary, IVIG admixture and administration involves several practical considerations. The admixture should conform to the product labeling, delivery should be prompt given the short stability, and administration should follow standard hospital procedure. Furthermore, clinical considerations include evaluating the patient’s past medical history and determining whether any adjustments to dosing or product selection are necessary in therapy.
CASE 2: Hemin in Acute Intermittent Porphyria
CC is a 49-year-old Caucasian female diagnosed with AIP approximately 15 years ago. She presents with the typical symptoms of porphyria, including nausea, vomiting, abdominal pain, and diarrhea during acute exacerbations. The common clinical features of the acute porphyrias in addition to CC’s complaints include tachycardia, constipation, and pain in the extremities. Abdominal pain is the most common clinical feature.20
AIP, one of four acute porphyria subtypes, is characterized by acute attacks of nonspecific gastrointestinal (GI) and neurological complaints. These symptoms are a result of accumulating heme precursors (porphyrins) due to alterations in the heme biosynthesis pathway.20 Strategies in the management of acute exacerbations include withdrawal of agents that increase heme demand (e.g., CYP450 inducers), supplementation with glucose, correction of electrolyte abnormalities, pain management, antiemetic therapy, hypertension management, and administration of hemin.21
CC’s management consists of supportive care, IV dextrose, and administration of hemin. IV dextrose is administered by alternating 1 liter of 5% dextrose/lactated Ringer’s solution and 1 liter 5% dextrose with 1 ampule (50 mL) of 50% dextrose at a rate of 200 mL/hour. The rate is titrated according to patient response. Additionally, hemin is administered at a dose of 4 mg/kg rounded to the nearest vial (313 mg; 1 vial of hemin) over a 10- to 15-minute infusion daily for 6 courses during this event. This is consistent with the hemin recommended dose of 1 to 4 mg/kg for 3 to 14 days based on the clinical signs.22 No more than 6 mg/kg of hemin should be given in any 24-hour period.22
Throughout the hospital course, electrolytes are monitored closely and replaced if necessary, pain is controlled using morphine, and nausea and vomiting are managed using serotonin 5-HT3 receptor antagonists. Agents that induce the hepatic CYP450 enzyme system are best avoided since the production of these enzymes is heme-dependent and increases heme demand.20 Symptoms respond well to treatment, with diarrhea subsiding first, followed by nausea and vomiting, and then abdominal pain.22
CC’s exacerbation requires a 7-day hospitalization. The typical duration of hospitalization ranges from 7 to 10 days, depending on the severity of exacerbation. The use of hemin appears to be appropriate in this patient based upon the pathologic role that depleted heme stores play. When infused, this biologic replenishes heme stores and prevents hepatic and/or marrow synthesis and accumulation of porphyrin metabolites, which contribute to symptoms.22
The use of hemin involves several practical issues, including timing of treatment, admixture, acquisition, and administration technique. Similar to IVIG, the dose must be administered soon after preparation. Reconstitution technique is also of importance due to the expense and availability of hemin. Another concern is product acquisition. Hospital pharmacies often do not stock hemin; therefore, practitioners should be provided outlets for acquiring the medication. Administration technique is also critical in order to minimize injection-site complications, including thrombophlebitis.
While the FDA-approved labeling of hemin states that a trial of hemin is appropriate after the patient has failed to respond to IV dextrose,22 several studies have demonstrated that early treatment (within 24 hours) with hemin improves outcomes.23-25 Under the guidance of the American Porphyria Foundation, a panel of experts suggested that early administration of hemin is critical in the management of an acute attack.20 In order to initiate early treatment, acquisition of hemin in a timely fashion is critical. Advising the department of pharmacy immediately upon arrival of a patient with known AIP is essential for ensuring the availability of hemin. Furthermore, utilization of the manufacturer’s 24-hour helpline may expedite drug acquisition.
Several key hemin admixture and administration issues are highlighted in TABLE 1. As with other biologics, hemin admixture has a relatively short-lived stability. Reconstituted hemin is a thick, black, opaque solution. The FDA-approved labeling indicates that hemin should be reconstituted in sterile water. The complex does not contain a bacteriostatic agent and undergoes rapid decomposition; thus reconstitution should occur immediately prior to administration.22 Reconstitution with albumin, although not FDA approved, might prolong stability and minimize thrombophlebitis.26 A heme-albumin solution may be prepared by slowly adding 132 mL of 25% albumin (using a vented needle) to a 313-mg vial of hemin. Gently swirl the vial to ensure homogeneity of the solution. The hemin concentration of the solution will be 2.4 mg/mL. The desired dose may be withdrawn and injected into an empty sterile bottle and protected from light using an amber bag.26 The dose should be rounded to the nearest vial size whenever possible to minimize waste. If hemin is reconstituted with albumin, the dose should be administered over at least 60 minutes.26
The issues involved with drug preparation and delivery to the patient in the setting of time constraints due to drug-stability concerns may be overcome by rapid diagnosis and health care practitioner communication. Unless the patient has a defined history of AIP, as in CC’s case, the diagnosis of this specific porphyria may be delayed and confounded by the diffuse GI symptomatology, pain, and neurologic symptoms seen in other conditions. A rapid diagnostic test, such as the Porphobilinogen (PBG) Test Kit, can aid in quickly identifying patients who may require and benefit from hemin.20
As with any time-critical agent, physicians, nurses, and pharmacists must communicate about meeting patient needs and preventing poor patient outcomes.27 The physician should inform the pharmacist of the intention to treat with hemin, the nurse should inform the pharmacist when he or she is ready to administer the dose, and the pharmacist must prepare and deliver the medication without delay. One recommendation is to have a protocol for preparation and/or administration as highlighted in the example in TABLE 2. A multidisciplinary team of pharmacists, physicians, and nurses should develop such protocols with approval from the hospital’s Pharmacy and Therapeutics Committee. Furthermore, members of the health care team that are most likely to treat these patients should be in-serviced to alleviate confusion and facilitate rapid access, preparation, and administration.
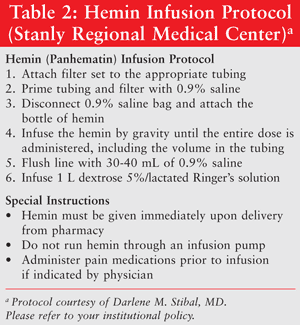
Hemin is generally well tolerated. One potential complication, thrombophlebitis, may be minimized by the use of indwelling catheters or the use of albumin as a diluent as discussed earlier. In the case of CC, a Hickman catheter is successful in preventing phlebitis. There is a potential for catheter occlusion, especially due to the physical properties (high viscosity) of the therapies for AIP—hemin and dextrose. This may be overcome by educating the nursing staff on proper catheter technique, which includes appropriately flushing the catheter after use and following institution protocols.
Difficulties with drug acquisition and high medication costs have the potential to delay proper treatment. To address these concerns, many manufacturers and disease-state organizations may provide guidance on procurement and reimbursement of medications. Hemin has a provider access line that guarantees delivery within 12 to 24 hours of request. Another option is product allocation, which is an invaluable service, given that many hospital pharmacies do not stock hemin. Additionally, to alleviate patient financial burden, patient reimbursement and assistance are often available by contacting the manufacturer.
CC’s AIP is successfully managed through avoidance of exacerbating factors (specifically CYP450 inducers that increase heme demand), IV dextrose, and hemin. To ensure the safe use of hemin, proper protocols should be developed (TABLE 2). Practitioners should be ready to obtain, prepare, and administer the appropriate medications in given situations. In-servicing staff and provision of protocols will help expedite processes, improve patient safety, and improve staff confidence. Inform patients of the availability of resources that can help ameliorate the financial burdens of therapy. Finally, if therapies require the use of indwelling catheters, educate patients on appropriate care.
CASE 3: Azacitidine (AZA) in Myelodysplastic Syndrome
KS is a 64-year-old male recently diagnosed with MDS, a group of diseases characterized by malformed bone marrow cells resulting in either abnormal blood counts or poorly functioning blood cells.28 One concern with MDS is the frequent (10%-40%) evolution of disease to acute myeloid leukemia (AML).29 Also posing a challenge is the predominance of this condition in the elderly.30 Organ function, particularly kidney and liver, is often altered in advanced age; therefore, drug dosage adjustments may be necessary to prevent toxicity. The National Cancer Institute’s Surveillance, Epidemiology, and End Report indicates that 86% of diagnosed MDS cases were in patients greater than or equal to 60 years of age (median 76 years). Additionally, a greater prevalence was noted in men (4.5 vs. 2.7 per 100,000).31 Common signs and symptoms of MDS, as seen in KS, include particularly progressive weakness, refractory anemia, spontaneous bruising, infection, and hemorrhage.32 Upon initial presentation, KS’s complete blood count is significant for neutropenia (1.5 × 103/mm3; 50% neutrophils, 4% blasts), decreased hemoglobin (6.3 mg/dL) and hematocrit (24%), and thrombocytopenia (27,000/mm3). A bone marrow biopsy reveals a hypercellular marrow with 20% blasts. A cytogenetic analysis is significant for trisomy 8.
The French-American-British (FAB) Classification System is one method used to categorize MDS and predict transformation to AML. The International Prognostic Scoring System (IPSS) also provides practitioners with median survival and predicts conversion to AML. Based on FAB, KS is diagnosed with refractory anemia with excess blasts (RAEB), which carries a high risk of transformation to AML.33 Furthermore, when staged utilizing the IPSS, KS is classified as intermediate-2 risk with a predicted median survival in the absence of therapy of 1.1 years.
Currently, the National Comprehensive Cancer Network MDS guidelines include DNA methyltransferase inhibitors (AZA or decitabine), lenalidomide, cyclosporine, and antithymocyte immunoglobulin as options for treating MDS.34 Treatment selection is based on patient risk level and goals of treatment.
Fenaux et al evaluated the efficacy of AZA (n = 179; 75 mg/m2 per day for 7 days every 28 days) versus conventional care (n=179; best supportive care, low-dose cytarabine, or intensive chemotherapy as selected by the investigators prior to randomization) in a phase III, multicenter, open-label, parallel-group, randomized, controlled trial.35 After a median follow-up of 21.1 months, median overall survival was significantly improved in the AZA group (24.5 months vs. 15.0 months; hazard ratio 0.58; 95% confidence interval 0.43-0.77; P = .0001). Further, based on Kaplan-Meier estimates, at 2 years, more patients in the AZA group survived compared with the conventional group (50.8% vs. 26.2%; P <.001).
The decision is made to treat KS with AZA (75 mg/m2 daily for 7 days, repeat cycle every 4 weeks) to reduce the risk of transformation and improve survival. AZA, a DNA methyltransferase inhibitor, is a nucleoside analog of cytidine that hypomethylates DNA in abnormal hematopoietic cells in the bone marrow.36 Additionally, AZA exhibits a direct cytotoxic effect on abnormal hematopoietic cells at higher doses.36 A total of 6 cycles are administered until an adequate response (i.e., improvement in hematocrit, platelets, red blood cells) is seen. The recommended starting dose for the first treatment cycle regardless of baseline hematologic laboratory values is 75 mg/m2 daily for 7 days. The dose may be prepared for administration by either the subcutaneous (SQ) or IV route (TABLE 1). Cycles should be repeated every 4 weeks for a minimum of 4 to 6 cycles. The dose may be escalated to 100 mg/m2 if no beneficial effect is seen and no toxicity other than nausea and vomiting has occurred after the second cycle.36 Although it is generally recommended to treat patients for a minimum of 4 to 6 cycles, patients often require additional treatment cycles to realize a complete or partial response to therapy. Treatment may be continued as long as it remains beneficial. Dosage adjustments may be necessary for renal dysfunction and/or hematologic laboratory results in subsequent cycles.
In addition, supportive care should be initiated and should encompass frequent observation and monitoring, psychosocial support, and quality of life assessment. In lower-risk patients, treatment goals may include hematologic improvement and reduction of transfusion requirements, while in higher-risk patients, improvements in overall and progression-free survival, as well as a reduction of transfusion requirements, may be important clinical outcomes.37 The ultimate goal of treatment is to alter the natural progression of disease.34
The standard 7-day dosing per cycle is often not schedule-friendly since it requires administration on weekends.38 In a recent open-label study, a 5-day cycle of AZA was both effective and safe.39 This may be an optimal strategy in patients who have transportation issues and may be unable to present for treatment on weekends. Although this treatment option was proven viable in the aforementioned study, the 5-day cycle is not FDA approved. KS is not given the 5-day cycle.
The use of AZA involves several practical issues. These include drug preparation, drug administration, and, most importantly, management of side effects. Although AZA is generally well tolerated, it is associated with myelosuppression, nausea and vomiting, and injection-site reactions. AZA is considered to be a moderately emetogenic chemotherapeutic agent.40,41 Thus, patients should be premedicated for nausea and vomiting with dexamethasone and 5-HT3 antagonists.40 In addition, the use of a neurokinin-1 antagonist may be considered. Side-effect management is critical since patients often require up to 6 cycles for optimal benefit. If patients are poorly managed, they may be less likely to complete the full treatment course. Furthermore, complications may be more likely to occur.
Preparation of the dose for SQ administration allows for storage under refrigeration for 8 hours (within 1 hour if not refrigerated). When prepared as an SQ injection, AZA presents as a slurry-like suspension. Allowing the suspension to return to room temperature prior to injection will ensure that the suspension equilibrates.36 As an IV dose, administration should be completed within 1 hour of reconstitution. In either case, prompt admixture and delivery should be a priority and requires close communication between pharmacists, nurses, and patients. An important consideration when deciding the appropriate route of administration is the volume of the dose. If the SQ dose volume exceeds 4 mL, the dose should be divided into two syringes; however, this may make the administration more challenging, and the IV route may be preferred. AZA is classified as a chemotherapeutic agent, and as such should be handled according to the safe handling of chemotherapy and cytotoxic agent policies at respective institutions. The drug dissolves quickly after reconstitution with sterile water and vigorous shaking. Verification of the appropriate technique in the product labeling is a rudimentary yet effective strategy, especially if difficulty arises during reconstitution.36
AZA therapy has been associated with improved survival of patients with MDS.35 Proper management of this therapy is critical to ensure patient adherence. As previously mentioned, the IV route may be preferred over the SQ route when larger doses are required. Practitioners must communicate which route is desired since preparation and administration techniques differ. Development of procedures that provide guidance on dosage adjustments for organ dysfunction, supportive care, and administration technique, in addition to patient education, may help facilitate medication understanding and safety.
Discussion
Several practical considerations are described in the above case discussions. Compliance with the product labeling for admixtures, stability, and administration is essential. Many of these products have a short shelf life once compounded, which must be taken into consideration. The development of institutional protocols for administration is critical to address product-specific issues. In addition, procedures for addressing patient specific issues, such as organ dysfunction, patient selection, and product acquisition, can benefit both the patient and the institution. Education of the staff should focus on improving the process, enhancing patient safety, and building staff confidence.
Conclusion
The cases discussed here illustrated three scenarios where hematologic agents were used successfully. In order to accomplish this, practitioners should be cognizant of caveats often associated with these products. This may involve recognition of the impact of comorbidities on therapy, appropriate admixture and administration techniques, and product acquisition. As new medications are introduced into the milieu of pharmacotherapeutic treatment options, it is crucial for practitioners to adopt a process that enables the recognition of treatment-dictating variables.
REFERENCES
1. Committee on Identifying and Preventing Medication Errors. In: Aspden P, Wolcott J, Bootman JL, Cronenwett LR, eds. Preventing Medication Errors: Quality Chasm Series. Washington, DC: National Academies Press; 2006.
2. American Society of Health-System Pharmacists. Proceedings of a summit on preventing patient harm and death from IV medication errors. Am J Health Syst Pharm. 2008;65:2367-2379.
3. Neylon AJ, Saunders PW, Howard MR, et al. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br J Haematol. 2000;122:966-974.
4. Idiopathic thrombocytopenic purpura. National Organization for Rare Disorders. www.raredisorders.org. Accessed November 24, 2008.
5. Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98:436-442.
6. George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3-40.
7. British Committee for Standards in Haematology. General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children, and in pregnancy. Br J Haematol. 2003;120:574-596.
8. Gammagard S/D (immune globulin intravenous [human]) package insert. Westlake Village, CA: Baxter Healthcare Corporation; October 2008.
9. Leong H, Stachnik H, Bonk ME, Matuszewski KA. Unlabeled uses of intravenous immune globulin. Am J Health-Sys Pharm. 2008;65:1815-1824.
10. Emerson GG, Herndon CN, Sreih AG. Thrombotic complications after intravenous immunoglobulin therapy in two patients. Pharmacotherapy. 2002;22:1638-1641.
11. Katz KA, Hivnor CM, Geist DE, et al. Stroke and deep venous thrombosis complicating intravenous immunoglobulin infusions. Arch Dermatol. 2003;139:991-993.
12. Go RS, Call TG. Deep venous thrombosis of the arm after intravenous immunoglobulin infusion: case report and literature review of intravenous immunoglobulin-related thrombotic complications. Mayo Clin Proc. 2000;75:83-85.
13. Traynor K. Baxter, Red Cross warn against rapid infusion of IGIV. April 22, 2002. www.ashp.org/import/news/
14. White DA, Leonard MC. Acute stroke with high dose intravenous immune globulin. Am J Health Syst Pharm. 2007;64:1611-1614.
15. Sokos DR, Berger M, Lazarus HM. Intravenous immunoglobulin: appropriate indications and uses in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:117-130.
16. Hefer D, Jaloudi M. Thromboembolic events as an emerging adverse effect during high dose intravenous immunoglobulin therapy in elderly patients: a case report and discussion of the relevant literature. Ann Hematol. 2005;84:411-415.
17. Katz U, Shoenfeld Y. Review: intravenous immunoglobulin therapy and thromboembolic complications. Lupus. 2005;14:802-808.
18. Knezevic-Maramica I, Kruskall MS. Intravenous immune globulins: an update for clinicians. Transfusion. 2003;43:1460-1480.
19. Dear Doctor Letter. Important drug warning: potential risk of acute renal failure reported to be associated with administration of immune globulin intravenous (human). September 24, 1999. www.fda.gov/
20. Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439-450.
21. Bonkovsky HL. Neurovisceral porphyrias: what a hematologist needs to know. Hematology Am Soc Hematol Educ Program. 2005;24-30.
22. Panhematin (hemin for injection) package insert. Deerfield, IL: Ovation Pharmaceuticals; June 2007.
23. Hift RJ, Meissner PN. An analysis of 112 acute porphyric attacks in Cape Town, South Africa: evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severity. Medicine (Baltimore). 2005;84:48-60.
24. Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyric attacks. Arch Intern Med. 1993;153:2004-2008.
25. Pierach CA. Hemin therapy for the porphyric attack. Semin Liver Dis. 1982;2:125-131.
26. Anderson KE, Bonkovsky HL, Bloomer JR, Shedlofsky SI. Reconstitution of hematin for intravenous infusion. Ann Intern Med. 2006;144:537-539.
27. Poor communication is common cause of errors. Healthcare Benchmarks Qual Improv. 2002;2:18-19.
28. Myelodysplastic syndromes. Aplastic Anemia & MDS International Foundation. www.aplastic.org/aplastic/
29. Saba HI. Myelodysplastic syndromes in the elderly. Cancer Control. 2001;8:79-102.
30. Kurzrock R. Myelodysplastic syndrome overview. Semin Hematol. 2002;39:18-25.
31. Rollison DE, Howlader N, Smith MT, et al. First report of national
estimates of the incidence of myelodysplastic syndromes and chronic myeloproliferative disorders from the US SEER program [abstract 247]. Blood. 2006;108:77a.
32. Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2002:136-161.
33. List AF, Doll FC. The myelodysplastic syndromes. In: Wintrobe’s Clinical Hematology. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:2320-2341.
34. The NCCN Clinical Practice Guidelines in Oncology. Myelodysplastic syndrome. Version 2.2010. National Comprehensive Cancer Network; 2009. www.nccn.org. Accessed September 24, 2009.
35. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223-232.
36. Vidaza (azacitidine for injection) package insert. Summit, NJ: Celgene Corporation; August 2008.
37. Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419-425.
38. List AF. Treatment strategies to optimize clinical benefit in the patient with myelodysplastic syndromes. Cancer Control 2008;15(suppl):29-39.
39. Lyons RM, Cosgriff T, Modi S, et al. Results of the initial treatment phase of a study of three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes (MDS) [abstract 819]. Blood. 2007;110:251a.
40. The NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version 3.2009. National Comprehensive Cancer Network; 2009. www.nccn.org. Accessed April 14, 2009.
41. Sullivan M, Hahn K, Kolesar JM. Azacitidine: a novel agent for myelodysplastic syndrome. Am J Health Syst Pharm. 2005;62:1567-1573.
To comment on this article, contact rdavidson@uspharmacist.com.






