ABSTRACT: Chronic kidney disease (CKD) is increasing in prevalence worldwide. Anemia is one of the most common complications of this disease. Luckily, it is also one of the most responsive to treatment. The use of iron and erythropoiesis-stimulating agents (ESAs) is the most effective means of treating the anemia of CKD. Proper knowledge of dosing these agents and managing their side-effect profiles is becoming essential for pharmacists.
The incidence of renal insufficiency and end-stage renal disease (ESRD) is on the rise in the United States. In 2006, the prevalence of ESRD was 506,256. From 1995 to 2006, a 3.6-fold increase in the prevalence of CKD was reported among patients covered by Medicare.1 As this patient population continues to grow, the importance of pharmacist knowledge about disease state–specific topics and pharmacotherapy is becoming essential.
One well-known complication associated with CKD and ESRD is anemia. As renal function declines, the incidence of anemia increases.2 Untreated anemia can significantly decrease a patient’s quality of life and can increase morbidity and mortality. Patients who are appropriately treated are relieved from fatigue and have improved cognitive functioning. There is also controversial evidence that treating anemia with ESAs in patients with CKD may aid in the preservation of cardiac and kidney function.3,4 However, further studies are needed to verify or refute this evidence and to support the beneficial effects of ESA therapy in this patient population.
Anemia in the setting of CKD or ESRD is primarily attributed to a lack of the hormone erythropoietin. In normal physiology, hypoxia stimulates erythropoietin synthesis and release by kidney cells. Erythropoietin serves to induce division and differentiation of progenitor cells in the bone marrow, in turn causing a release of immature red blood cells (known as reticulocytes) into the blood stream where they mature. In patients with renal insufficiency, there is a lack of erythropoietin production and an inappropriate response to hypoxia, resulting in a failure to produce sufficient mature red blood cells (RBCs). In addition to decreased erythropoietin, patients with CKD may have other factors contributing to anemia. This includes a shortened life span of RBCs in the presence of uremia; deficiencies in iron, folic acid, and vitamin B12; and blood loss due to frequent laboratory draws as well as loss during hemodialysis.
Evaluating Labs
The National Kidney Foundation’s 2006 Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines for anemia of CKD recommend that hemoglobin (Hgb) testing be done annually in all patients with CKD.5 The diagnosis of anemia is made and further evaluation is initiated when the hemoglobin (Hgb) level is <12 g/dL in adult females or <13.5 g/dL in adult males.5 The workup includes a complete blood count (CBC) with differential and platelet count, absolute reticulocyte count, serum ferritin, and serum transferrin saturation (TSAT) or content of hemoglobin in reticulocytes (CHr). In addition, testing for blood in stool may be considered. Nutritional deficiencies such as folate and/or vitamin B-12 deficiency may be detected from RBC indices or direct testing of serum levels. Vitamin supplements are given if indicated. Any other factors that may contribute to anemia must be evaluated and treated prior to initiation of therapy with ESAs.
Similar to anemia of chronic disease, CKD-related anemia is usually normocytic and normochromic. Thus, the serum ferritin is utilized to evaluate body iron stores, while the TSAT and CHr tests are utilized to evaluate the availability of iron for erythropoiesis. The NKF KDOQI targets for patients with hemodialysis-dependent CKD are serum ferritin >200 ng/mL and TSAT >20% or CHr >29 pg/cell.5 The targets for patients with predialysis CKD and peritoneal dialysis are serum ferritin >100 ng/mL and TSAT >20%.4 An upper level of ferritin is not defined in the KDOQI guidelines; however, when the serum ferritin level is >500 ng/mL, clinicians should take into consideration ESA responsiveness, Hgb and TSAT levels, and clinical status of the patient when deciding whether to administer IV iron.5 Serum ferritin is an acute-phase reactant and levels increase in response to inflammation, which is commonly present in many patients undergoing hemodialysis. It is important to evaluate both the TSAT and ferritin levels along with the hemoglobin value when determining whether to administer iron in a patient on ESA therapy. The presence of inflammation and/or infection and an increased serum ferritin does not necessarily preclude the administration of concomitant iron therapy.
Functional iron deficiency occurs when there is adequate iron stores as evidenced by normal iron status parameters; however, the body is unable to mobilize enough iron from the liver and other storage sites to adequately support erythropoiesis with ESAs. In the Dialysis Patient's Response to IV Iron with Elevated Ferritin (DRIVE) and DRIVE-II studies, the administration of up to 1 g of IV iron in patients with anemia undergoing hemodialysis on adequate ESA therapy with ferritin values ≤1,200 ng/mL and low TSAT values was shown to improve hemoglobin and decrease ESA dose.6,7
Iron Replacement
Inadequate iron stores or decreased availability of iron is the most common reason for ESA hyporesponsiveness or resistance. Therefore, it is essential that iron deficiency be treated prior to starting ESA therapy and that sufficient iron stores are maintained during therapy. As defined by NKF KDOQI, the goals of iron therapy in the patient with CKD are to “avoid storage iron depletion, prevent iron-deficient erythropoiesis, and achieve and maintain target hemoglobin levels.”5 Iron replacement can be given with oral or parenteral products. Several studies have shown that IV administration of iron is superior to oral iron administration in patients on ESAs.8-10 Oral iron therapy is limited by many factors, including poor patient compliance due to gastrointestinal (GI) side effects, suboptimal absorption, and drug–drug interactions. For best absorption, it is recommended that oral iron be taken on an empty stomach; however, this increases the incidence of GI distress. Advise patients to take oral iron supplements with food if stomach upset occurs, but to avoid dairy products, eggs, fiber, bran, antacids, and vitamin products within 2 hours of taking the iron supplement. Counsel patients on potential drug–drug interactions between oral iron supplements and certain antibiotics and to alert their prescriber when receiving a prescription for antibiotic therapy. Patients on gastric acid suppression therapy may not respond well to oral iron therapy due to poor absorption. Because of its low cost and ease of administration, a trial of oral iron therapy of at least 200 mg of elemental iron per day may be considered in patients with predialysis CKD and who are on peritoneal dialysis. In patients with hemodialysis-dependent CKD, the preferred route of iron administration is intravenous.5
There are currently four injectable iron preparations available in the U.S.: iron dextrans (INFeD, DexFerrum), iron sucrose (Venofer), sodium ferric gluconate (Ferrlecit), and ferumoxytol (Feraheme). All are highly efficacious, but they differ with respect to indication, dosing, and side effect profile.11-14
The iron dextran preparations are FDA approved for the treatment of iron deficiency anemia in adults and children older than 4 months of age and are approved for both intramuscular (IM) and IV use.11 Iron sucrose and ferumoxytol are FDA approved for IV use in the treatment of iron deficiency anemia in adult patients with CKD.12,14 Sodium ferric gluconate’s FDA-approved use is limited to the treatment of iron deficiency anemia in adult and pediatric patients aged 6 years and older on chronic hemodialysis receiving epoetin.13
Most patients with CKD with iron parameters below NKF KDOQI goals will require a minimum dose of 1,000 mg of elemental iron for iron store replenishment and to raise their hemoglobin.12 The dosing regimens for each iron product varies (TABLE 1). Once low iron stores are replenished, a maintenance dose of IV iron is usually required to maintain iron parameters within established guidelines and support erythropoiesis with ESA therapy. This may be accomplished with low doses of IV iron given once weekly to once monthly.
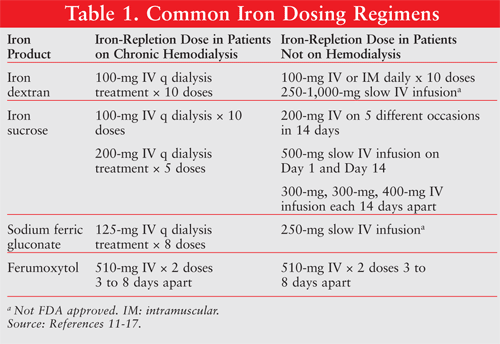
Severe hypersensitivity reactions have been reported with all of the parenteral iron products. Life-threatening adverse drug events (ADRs) have occurred most frequently with the iron dextran products, most notably with the high molecular weight iron dextran, DexFerrum.18 Both iron dextran products carry a black box warning describing the risk for anaphylactic-type reactions or death and the need for administering a test dose of iron dextran prior to the first therapeutic dose with careful monitoring of the patient for signs or symptoms of an anaphylactic-type reaction.11 Iron sucrose, sodium ferric gluconate, and ferumoxytol appear to be safer than the iron dextran products, with significantly fewer reported severe hypersensitivity or nonlife-threatening ADRs.12-14 The administration of a test dose is not required with these products. The reported rates of serious hypersensitivity reactions for each product are as follows: in about 1 in 150 patients receiving iron dextran, in about 1 in 575 patients receiving ferumoxytol, in about 1 in 1,000 patients receiving sodium ferric gluconate, and in <1 in 1,000 patients receiving iron sucrose.11-14 Other side effects of nondextran iron products include hypotension, dizziness, and headache, especially when infused too rapidly or in too large of a dose.
When choosing an injectable iron product, route of administration, total iron requirement, convenience, and patient accessibility to health care services are all taken into consideration. Iron dextran products are unique in that they allow for IM administration and total dose infusion of iron therapy; however, these products carry the highest risk of life-threatening ADRs.11 Newer iron products such as iron sucrose and sodium ferric gluconate have better safety profiles than iron dextrans and therefore are preferred for patients who are hemodialysis dependent.12,13 However, they are limited by the need to administer smaller doses, which may be challenging in patients with CKD who are not on hemodialysis and in patients undergoing peritoneal dialysis who require iron-replenishment dosing. Ferumoxytol may be preferred in this setting due to the ability to administer a larger iron dose by rapid IV injection, thus requiring only two clinic visits.14
Erythropoiesis-Stimulating Agents
The advent of ESAs has dramatically improved the treatment of anemia in patients with renal disease. These injectable medications are proteins that are analogous to endogenous erythropoietin. In the U.S., the available ESAs include darbepoetin alfa (Aranesp) and epoetin alfa (Procrit, Epogen). The products are similar in action and side-effect profiles but differ in dosing regimens. Initial dosing for darbepoetin alfa is 0.75 mcg/kg IV or subcutaneous (subcut) every 2 weeks in patients not on dialysis and 0.45 mcg/kg weekly in patients with ESRD.19 Initial dosing for epoetin alfa is 50 to 100 units/kg subcut or IV three times a week for patients with ESRD.20,21 The usual dose of epoetin alfa required for patients not on dialysis ranges from 75 to 150 units/kg per week.19,20 The dose of ESA required to reach target Hgb levels varies widely and depends on initial Hgb, patient response, and clinical circumstances.
ESA therapy is initiated when the hemoglobin level is <11 g/dL and other causes of anemia have been ruled out or treated.5 This is particularly important in patients with CKD who are not on hemodialysis, as the cause of anemia is often a process of elimination, leaving anemia of renal disease as the last option. These patients must be screened to rule out other causes such as bleeding and nutritional deficiencies. In contrast, anemia due to renal disease in patients with ESRD is nearly universal, and therefore a more thorough work-up may not be as essential.
Prior to initiating ESA therapy, patients must also be screened for appropriateness and contraindications. The medication may cause an increase in blood pressure, which may precipitate hypertensive encephalopathy and seizures, so blood pressure must be adequately controlled. Blood pressure should be closely monitored throughout therapy, particularly during the initial phase as Hgb is rising. Antihypertensive medications should be adjusted accordingly, and compliance should be heavily emphasized to all patients receiving ESAs. Other contraindications include known hypersensitivities to mammalian cell-derived products and human albumin.19-21
There are a variety of possible ADRs to monitor. One rare, but dangerous, reaction is pure red cell aplasia (PRCA). Therapy must be stopped in any patient who develops a sudden loss of response to an ESA in combination with low reticulocyte count. The manufacturer should be notified, and the patient should be tested for ESA-neutralizing antibodies. If the test is positive, the patient should never be restarted on any ESA. Some of the more commonly experienced adverse events include hypertension, headache, tachycardia, nausea/vomiting, shortness of breath, hyperkalemia, and diarrhea. Hypersensitivity reactions have been reported. If a patient develops a rash or itching, the symptom may be treated and therapy can be continued. However, in the incidence of a severe anaphylactic reaction, therapy must be permanently discontinued.19-21
The FDA has issued a black box warning for patients with renal disease on ESA therapy, indicating that targeting high Hgb levels with ESAs increases the risk of death and other cardiovascular events. The risk of venous thromboembolism (VTE) is highest in patients with a targeted Hgb >13 g/dL and in those patients who have a rapid rise in Hgb. Therefore, it is recommended to use the lowest dose of ESA that will gradually increase the hemoglobin concentration to the lowest level sufficient to avoid the need for RBC transfusions. The NKI KDOQI guidelines suggest a target Hgb range of 11 to 12 g/dL.5 Achieving and maintaining Hgb in therapeutic range require diligent monitoring and dose adjustments. When patients are first initiated on therapy, the Hgb should be monitored every 2 weeks. Once a patient is stabilized, testing may be extended to every month. If the Hgb has increased by more than 1 g/dL in any 2-week period or if the Hgb has reached or exceeds 12 g/dL, the ESA dose should be reduced by 25%. Withhold the ESA dose only if clinically necessary. Dose reductions are the preferred method when the hemoglobin is above goal range or is rising too rapidly. The length of time between doses may also be extended as a way of achieving Hgb levels within goal range. If the increase in Hgb is less than or equal to 1 g/dL over 4 weeks, the ESA dose should be increased by 25%.19-21 Dose increases should not be made more frequently than every 2 weeks, as it will take at least this long for a new start or dose adjustment to achieve maximum efficacy.5 Frequent monitoring is crucial to ensuring proper treatment while minimizing potential ADRs.
If the hemoglobin is inappropriately low for the ESA dose administered after 4 to 6 weeks of treatment, evaluate for causes of hyporesponsiveness.5 Consider underlying infectious, inflammatory, or malignant processes; occult blood loss; underlying hematologic diseases; vitamin deficiencies; hemolysis; aluminum intoxication; osteitis fibrosa cystica; and PRCA as possible causes. It is usually appropriate to continue the same ESA dose regimen while correcting the cause of ESA resistance.
The preferred route of administration of ESAs in patients with CKD predialysis and patients on peritoneal dialysis is subcut as it takes advantage of the prolonged half-life of ESAs. The IV route is preferred in patients on chronic hemodialysis therapy due to ease of administration. When a patient stabilized on epoetin alfa therapy is converted from IV administration to subcut administration, the epoetin alfa dose may need to be reduced by 25% to 30% to maintain the same hemoglobin.5 When converting patients from darbepoetin alfa to epoetin alfa, pharmacists can refer to the conversion table provided in the darbepoetin alfa package insert.
Adjuvant Therapy
The use of ascorbic acid to treat functional iron deficiency in patients with anemia of kidney disease is currently under investigation. Several small studies have shown that using 100 to 500 mg of IV vitamin C three times a week can improve iron utilization as displayed by decreased ferritin, increased TSAT, and improvements in Hgb.22-24 However, due to the small number of studies currently available and the lack of safety information, particularly in regards to the risk of oxalosis, NKF KDOQI does not currently recommend this treatment.5
Carnitine, a compound biosynthesized in the liver and kidneys from the amino acids lysine or methionine, is required for the transport of long-chain fatty acids into the mitochondria and is important in energy metabolism. Patients undergoing hemodialysis may suffer from carnitine deficiency due to loss of carnitine during dialysis.25,26 L-carnitine supplementation of 20 mg/kg IV in patients with ESRD who are on hemodialysis is thought to improve anemia; however, there is not enough evidence to support recommending its use in all patients with CKD who are anemic.27-29 NKF KDOQI does not recommend the use of carnitine in the management of anemia in patients with CKD due to the absence of high-quality evidence for efficacy and safety.5
Pharmacist’s Role
Health care costs have increased substantially over the past several years, creating the need to optimize health care resources in order to provide the most cost-effective and beneficial care in the treatment of the patient with anemia of CKD. Pharmacists have a critical role in ensuring the delivery of safe and effective treatment to these patients. At a minimum, pharmacists who have access to patient laboratory values should ensure that upon dispensing an ESA, the Hgb level is not above goal range or that the dose has been appropriately decreased to prevent the risk of VTE. Pharmacists may also play a key role in identifying patients with anemia with early stages of CKD who may benefit from ESA and iron therapy and discussing with the provider if initiation of treatment is appropriate.
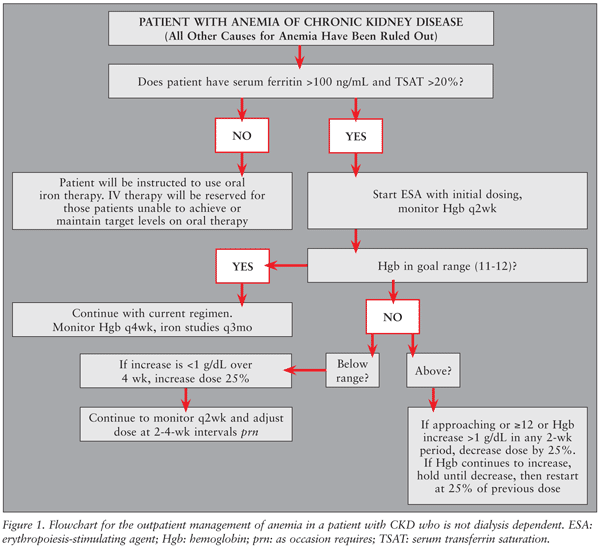
Pharmacists may take more active roles by becoming involved in anemia management services. Outpatient clinics help to ensure that patients with predialysis CKD are receiving appropriate therapy, dose adjustments, and follow-up. Pharmacists can enter into collaborative practices in which they are involved in point-of-care Hgb testing, determining and administering ESA doses, and reviewing and prescribing iron therapy, as well as educating patients about anemia and the importance of compliance. A typical flowchart for the management of anemia of CKD in a patient who is nondialysis dependent in the outpatient clinic setting is presented in FIGURE 1. In a study published by Bucaloiu et al, the outcomes of patients being treated by a pharmacist-managed outpatient clinic were compared to those receiving care from PCPs. The pharmacist clinic was more economical, using less ESA, but achieved a statistically significant advantage in clinical outcomes in terms of time to achievement of goal Hgb and maintaining a higher percentage of Hgb and TSAT values in target range.30
Inpatient pharmacist anemia management services are also becoming more prevalent. The pharmacist can serve as the link between outpatient and inpatient anemia therapy. By ensuring appropriate medication reconciliation, inpatient anemia management pharmacists carry out NKF KDOQI’s recommendation that “administration in ESA-dependent patients should continue during hospitalization.”5 These pharmacists are aware of factors that can specifically affect Hgb and iron in an acute setting, such as procedures, infection, and clinical patient status, and work to assure that inpatient anemia therapy is optimized. Pharmacists can also assist providers in choosing the correct dose of ESA when converting a patient from one ESA form to another. These services are also cost effective, as documented at our institution. With pharmacist management, therapeutic hemoglobin goals were achieved or maintained utilizing less epoetin alfa and with fewer serious adverse events when compared to traditional prescriber-managed dosing.31
REFERENCES
1. National Institutes of Health, National Institute of Diabetes and Diges-tive and Kidney Diseases. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health; 2008.
2. McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501-1510.
3. Mohanram A, Zhang Z, Shahinfar S, et al. Anemia and end-stage renal disease patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66:1131-1138.
4. Levin A, Thompson CR, Ross H, et al. Prevalent left ventricular mass
index in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125-134.
5. National Kidney Foundation. KDOQI clinical practice guidelines and
clinical practice recommendations for anemia in chronic kidney disease.
Am J Kidney Dis. 2006;47(suppl 3):S111-S145.
6. Coyne DW, Kapoian T, Suki W, et al, and the DRIVE Study Group.
Ferric gluconate is highly efficacious in anemic hemodialysis patients
with high serum ferritin and low transferring saturation: results of
the Dialysis Patients’ Response to IV Iron with Elevated Ferritin
(DRIVE) Study. J Am Soc Nephrol. 2007;18:975-984.
7. Kapoian T, O’Mara NB, Singh AK, et al. Ferric gluconate reduces
epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19:372-379.
8. Macdougall IC, Tucker B, Thompson J, et al. A randomized controlled
study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996;50:1694-1699.
9. Wingard RL, Parker RA, Ismail N, et al. Efficacy of oral iron
therapy in patients receiving recombinant human erythropoietin. Am J Kidney Dis. 1995;25:433-439.
10. Fudin R, Jaichenko J, Shostak A. Correction of uremic iron deficiency anemia in hemodialyzed patients: a prospective study. Nephron. 1998;79:299-305.
11. INFeD (iron dextrant) package insert. Morristown, NJ: Watson Pharma, Inc; September 2009.
12. Venofer (iron sucrose) package insert. Shirley, NY: Amercan Regent, Inc; October 2008.
13. Ferrlecit (sodium ferric gluconate) package insert. Corona, CA: Watson Pharma, Inc; September 2006.
14. Feraheme (ferumoytol) package insert. Lexington, MA: AMAG Pharmaceuticals Inc; June 2009.
15. Auerbach M, Winchester J, Wahab A, et al. A randomized trial of
three iron dextran infusion methods for anemia in EPO-treated dialysis
patients. Am J Kidney Dis. 1998;31:81-86.
16. Dahdah K, Patrie JT, Bolton WK. Intravenous iron dextran treatment in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000;36:775-782.
17. Folkert VW, Michael B, Argarwal, et al. Chronic use of sodium
ferric gluconate complex in hemodialysis patients: safety of
higher-dose (> or = 250 mg) administration. Am J Kidney Dis. 2003;41:651-657.
18. Chertow GM, Mason PD, Vaage-Nilsen O, Almen J. Update on adverse drug events associated with parenteral iron. Nehprol Dial Transplant. 2006;21:378-382.
19. Aranesp (darbeportin) package insert. Thousand Oaks, CA: Amgen; April 2009.
20. Epogen (epoetin alfa) package insert. Thousand Oaks, CA: Amgen; April 2009.
21. Procrit (epoetin alfa) package insert. Thousand Oaks, CA: Amgen; April 2009.
22. Lin CL, Hsu PY, Yang HY, Huang CC. Low dose intravenous ascorbic
acid for erythropoietin-hyporesponsive anemia in diabetic hemodialysis
patients with iron overload. Ren Fail. 2003;25:445-453.
23. Giancaspro V, Nuzziello M, Pallotta G, et al. Intravenous ascorbic
acid in hemodialysis patients with functional iron deficiency: a
clinical trial. J Nephrol. 2000;13:444-449.
24. Tarng DC, Wei YH, Huang TP, et al. Intravenous ascorbic acid as an
adjuvant therapy for recombinant erythropoietin in hemodialysis
patients with hyperferritinemia. Kidney Int. 1999;55:2477-2486.
25. Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am J Kidney Dis. 2003;41(suppl 4):S13-S26.
26. Bain MA, Faull R, Milne RW, et al. Oral L-carnitine: metabolite formation and hemodialysis. Curr Drug Metab. 2006;7:811-816.
27. Eknoyan G, Latos DL, Lindberg J. Practice recommendations for the
use of L-carnitine in dialysis-related carnitine disorder. National
Kidney Foundation Carnitine Consensus Conference. Am J Kidney Dis. 2003;41:868-876.
28. Hurot JM, Cucherat M, Haugh M, et al. Effects of L-carnitine
supplementation in maintenance hemodialysis patients: a systematic
review. J Am Soc Nephrol. 2002;13:708-714.
29. Steinman TI, Nissenson AR, Glassock RJ, et al. L-carnitine use in
dialysis patients: is national coverage for supplementation justified?
What were CMS regulators thinking—or were they? Nephrol News Issues. 2003;17:28-30,32-34,36 passim.
30. Bucaloiu ID, Akers G, Bermudez MC, et al. Outpatient erythropoietin
administered through a protocol-driven, pharmacist-managed program may
produce significant patient and economic benefits. Manag Care Interface. 2007;20:26-30.
31. Moriarity-Suggs C, Gann N. Cost savings derived from an inpatient
pharmacist driven anemia management protocol. Presented at the
California Society of Health Systems Pharmacists Seminar; October 9-12,
2008; Anaheim, CA.
To comment on this article, contact rdavidson@uspharmacis.com.






