US Pharm. 2010;35(6)(Generic Drug Review suppl):8-9.
Access to affordable drugs is critical to the well-being of the general population. By the same token, patient safety is helped, not hurt, by generic versions of a formulation that contain the same active pharmaceutical ingredient (API). The FDA permits interchangeability of brand-name and generic biopharmaceuticals so long as the quality and performance of the generic remain the same as those of the brand-name drug. The FDA evaluated 2,070 studies conducted between 1996 and 1997 that compared the absorption of brand-name and generic drugs into the human body. These studies were submitted to the FDA to support the approval of generics. The average difference in absorption between generic and brand-name drugs was 3.5%. Some generics were absorbed slightly more or slightly less; nevertheless, the FDA’s determination in this regard is final and binding.
Pricing: There is a big difference in cost between generic and brand-name drugs. On average, the cost of a generic drug is 80% to 85% lower than that of the brand-name drug. Several independent researchers have shown that total prescription drug expenditures increased by 4% from 2006 to 2007 and then increased by 3.2% from 2007 to 2008, substantially less than the 8.9% growth rate from 2005 to 2006. One factor cited as a reason for the slowdown is the increase in the availability and use of generic drugs. Generic manufacturers are able to sell their products for a lower price—not because the products are of lesser quality, but because the manufacturers generally do not engage in costly advertising, marketing, and promotion or significant research and development.
FDA Approval Process: The FDA maintains and updates a list of drug products for which a completed ANDA (Abbreviated New Drug Application) has been received by the Office of Generic Drugs (OGD). In 2007, the number of ANDA submissions to the OGD peaked at 880. The median approval time for ANDAs was 21.1 months in 2008, which is a 29% increase over the lowest approval time of 16.3 months in 2004.
Therapeutic Equivalence: Consumers have expressed concern as to whether the safety and efficacy profile of a drug could change if a switch were made from a brand-name product to an FDA-designated therapeutically equivalent generic product. In response, the FDA has required that all drugs marketed in the United States meet specifications for identity, strength, quality, purity, and potency. Additionally, the FDA requires rigorous tests and procedures to ensure that the generic drug is interchangeable with the brand-name drug for the approved indications. For these reasons, the FDA-approved product label does not recommend that additional testing be performed by health care practitioners (HCPs) when a switch is made from a brand-name drug to an equivalent generic drug product. Brand-name drug products and therapeutically equivalent generic drug products are identified in the FDA publication Approved Drug Products with Therapeutic Equivalence Evaluations (also known as the Orange Book). To date, there are no documented examples of a generic product manufactured to meet its approved specifications that could not be used interchangeably with the corresponding brand-name drug. The FDA may ask the manufacturers to perform additional tests for approval of both the brand-name and the generic product, depending on the complexity of the drug and also on whether small changes in the dose and/or blood concentration could result in changes in the drug’s efficacy or safety.
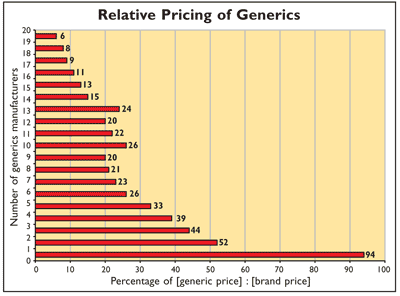
Generic Competition: Generic competition is associated with a lower drug price, with the entry of the second generic competitor being associated with the largest price reduction (see chart). This conclusion is based on FDA analyses of retail sales data from IMS Health based on the computation for the same API and dosage form, but ignoring the differences in strength and package sizes. Monthly price per dose was computed for each brand-name drug; for each generic, the average monthly price per dose and the number of generics were calculated. These prices were combined by drug and by the ratio of the average generic price to the corresponding branded product’s price. Excluded were products for which both branded and generic sales did not reach at least 1,000 doses per month. Ratios were grouped according to the number of generic manufacturers and the computed average of the individual price ratios. On average, the first generic competitor prices its product only slightly lower (6%) than the brand-name manufacturer. However, the appearance of a second generic manufacturer reduces the average generic price to 52% of the brand-name price. As additional generic manufacturers market the product, the price continues to fall, but more slowly. For drugs that attract a large number of generic manufacturers, the average generic price falls to 20% or lower of the branded price.
Commentary: Until recently, many brand-name drug manufacturers invested a substantial amount of their research and marketing dollars in the development of blockbuster drugs, only to yield their intellectual property to lower-priced generic competitors once the patents expired. But now, with $90 billion in brand-name drug sales in danger of being lost to generic competition over the next few years, some brand-name drug manufacturers are selling generics to offset revenue declines through postpatent profits from the innovative drugs they developed. More money can be charged for company-branded generics because of the promise of quality. See SIDEBAR 1 for examples of steps taken to offset declining brand-name drug sales.

Branded generics may appeal to leading drugmakers because they represent a hybrid of generic and brand-name models, allowing their existing commercial distribution system and marketing skills to sell premium-priced generics as if they were brand-name drugs. As government health care programs and health insurers in emerging markets develop further, consumers may be encouraged or required to switch from midpriced branded generics to low-cost, no-name generics, but this could take time to evolve.
To comment on this article, contact rdavidson@uspharmacist.com.






