Some of the drugs that are approved and distributed in the United States are either fully manufactured overseas or are manufactured within the U.S. using foreign-made ingredients. These drugs are distinguished from counterfeit or illegally imported products, which are made illegally to unlawfully duplicate an approved product or are brought illegally into the U.S. from another country (such as Mexico or Canada) with the intention of creating profit. The products may be legally approved in other countries, but they lack the approval of the FDA. This article discusses FDA-approved drug products that are manufactured overseas.
The FDA inspects domestic and foreign establishments prior to approving any new drug product to be marketed in the U.S. This action is triggered by the submission of a New Drug Application (NDA) or an Abbreviated New Drug Application (ANDA). The FDA is charged with inspecting each establishment every 2 years for compliance with Good Manufacturing Practice (GMP) surveillance postapproval standards. When the FDA identified that it did not have the resources necessary to meet this requirement, it developed a risk-based process to select which establishment it would inspect.1
Foreign-Made Drugs on the Rise in the U.S.
The number of foreign-made drug products in the U.S. is increasing rapidly. In 2009, imports regulated by the FDA had doubled since 2004.2 All drugs approved in the U.S., regardless of where they are made, must be in compliance with the Federal Food, Drug, and Cosmetic Act (FD&C), which requires that drugs meet manufacturing standards to assure quality and product label requirements.
The FDA assigns inspectors to key locations across the globe to help maintain the quality of imported drug products. The FDA has posts in China, India, the Middle East, Europe, and Latin America.3 The China office opened in November 2008, with the office in India opening soon after, in December of the same year. The Latin American offices, which are located in Costa Rica, Chile, and Mexico, opened in 2009. Europe holds FDA offices in England, Belgium, and Italy. The FDA’s Office of International Programs in Rockville, Maryland, leads all international regulatory operations, including those in Canada, Australia, New Zealand, Africa, Asia, and the Middle East. The FDA also collaborates with foreign regulatory agencies to discuss relevant information about products.3
Why do we have so many offices in foreign countries? In November 2007, the U.S. Government Accountability Office (GAO) testified on weaknesses in the FDA’s foreign drug-inspection program.4 It had been 9 years since the last report, prompting an inquiry. The GAO released preliminary findings in 2008 on how the FDA had been working towards addressing the identified weaknesses.
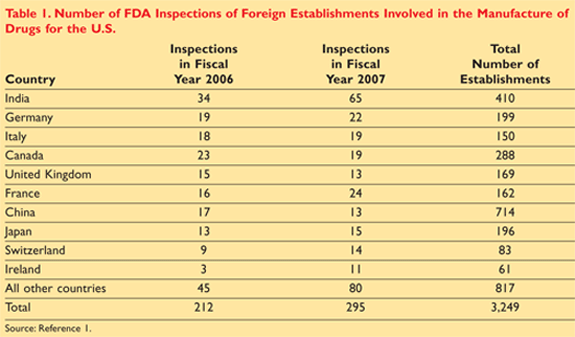
The FDA has made progress in conducting more foreign inspections, but it still inspects relatively few establishments. The FDA conducted more foreign-establishment inspections in fiscal year 2007 than it had in each of the 5 previous fiscal years. However, the agency still inspected fewer than 11% of the 3,249 foreign establishments on the prioritized list of locations that it used to plan its fiscal year 2007 GMP surveillance inspections.4
Additional statistics on the subject are shown in TABLE 1. The report states that it would take $67 to $71 million each year to inspect these establishments biennially. The FDA dedicated $10 million to foreign inspections in 2007. According to FDA budget documents, the agency estimates that it would only dedicate a total of about $11 million in fiscal year 2008 and $13 million in fiscal year 2009 to all foreign inspections.
The official position of the Generic Pharmaceutical Association (GPhA) on this matter encourages three principles5: strengthening FDA funding to allow for more inspections; establishing one uniform, high-quality inspection system for both domestic and foreign facilities to ensure that all countries are adhering to the same standards held in the U.S.; and implementing a “risk-based” approach so that more attention is focused on those facilities at greatest risk.
In a press release on the same topic, the GPhA assures the public that the industry holds all suppliers, including active-ingredient suppliers, to the highest standards.6 It states that its member companies conduct extensive auditing of suppliers; validation testing of incoming materials; and strict due-diligence programs to comply with FDA regulations.
Research Lacking on Foreign Drug Quality
A review of the literature implies that few published studies with statistics on foreign product quality are accessible for public viewing. A pilot study published in 2009 was launched in two cities in India and showed certain data that suggested substandard pharmaceutical product quality found in these two cities.7 However, the study showed that the production of substandard drugs occurred in a subset of manufacturers, specifically those that lacked a proper regulatory environment. An abstract published in the East African Medical Journal in 2004 was conducted to establish the quality of pharmaceutical products manufactured by the respective industries in Kenya and determine whether manufacturing practices also affected the outcome.8 This study is very limited, as it was only a questionnaire of policies; no drugs or products were actually tested. The questionnaire-based study did imply that the ratio of qualified staff to product range produced seemed to have an influence on quality.
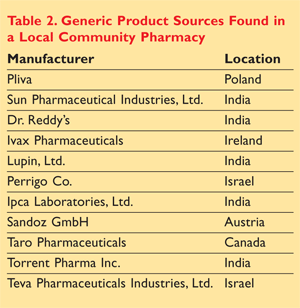
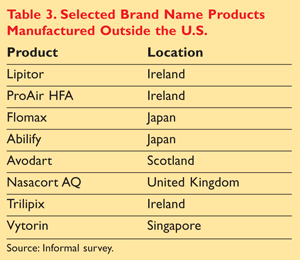
After a walk-through of generic prescription medications stocked in a local community pharmacy, we noted that many of the products that bear a distributor label of U.S. origin are actually often manufactured in foreign countries. This list is by no means all-inclusive, but TABLE 2 offers insight into the generic product sources in one pharmacy. One must not forget that generic products are not the only products manufactured in foreign countries. Selected brand name drug products that are manufactured overseas are listed in TABLE 3.
Both generic and brand name manufacturers are required to ensure that their overseas suppliers maintain good practices and standards to ensure quality products. We often hear more complaints against generic products. This is most likely due to the fact that there are approximately twice the number of FDA-approved generic products as there are brand name products.9 This is a complex issue, but is nonetheless of the utmost importance. Budgetary and manpower constraints have caused a lack of oversight in foreign facilities. Based on the increase in foreign FDA posts, it appears the agency has prioritized in solving this issue. As seen in the GAO report discussed earlier, however, it will take a much larger budget to ensure 100% compliance.
REFERENCES
1. Drug safety: preliminary findings suggest weaknesses in FDA’s program for inspecting foreign drug manufacturers. Washington DC: Government Accountability Office; November 1, 2007. Publication GAO-08-224T. http://gao.gov/products/GAO-08-224T. Accessed February 27, 2010.
2. About FDA: how does FDA oversee domestic and foreign drug manufacturing? Silver Springs, MD: U.S. Food and Drug Administration. www.fda.gov/AboutFDA/Basics/ucm194989.htm. Accessed February 1, 2010.
3. FDA’s international posts: improving the safety of imported food and medical products. Silver Springs, MD: U.S. Food and Drug Administration; October 20, 2009. www.fda.gov/ForConsumers/ConsumerUpdates/ucm185769.htm. Accessed February 1, 2010.
4. Drug safety: preliminary findings suggest recent FDA initiatives have potential, but do not fully address weaknesses in its foreign drug inspection program. Washington, DC: Government Accountability Office; April 22, 2008. Publication GAO-08-701T. http://goa.gov/products/GAO-08-701T. Accessed February 4, 2010.
5. GPhA position. Arlington, VA: Generic Pharmaceutical Association. www.gphaonline.org/print/issues/foreign-inspections reimportation. Accessed February 1, 2010.
6. FDA standards and foreign inspections. All medicines should follow the gold standard adhered to by the highly regulated brand and generic pharmaceutical industries. Press release. Arlington, VA: Generic Pharmaceutical Association. www.gphaonline.org/print/media/press-releases/2009/02/12/fda-
standards-and-foreign-inspections. Accessed February 1, 2010.
7. Bate R, Tren R, Mooney L, et al. Pilot study of essential drug quality in two major cities in India. PLoS One. 2009;4:e6003.
8. Orwa JA, Keter LK, Ouko SP, et al. Influence of manufacturing practices on quality of pharmaceutical products manufactured in Kenya. East Afr Med J. 2004;81:287-292.
9. Okie S. Multinational medicines—ensuring drug quality in an era of global manufacturing. N Engl J Med. 2009;361:737-740.
To comment on this article, contact rdavidson@uspharmacist.com.






