US Pharm. 2010;35(7)(Oncology suppl):10-13.
ABSTRACT: Approximately 1,100 new cases of cutaneous T-cell lymphoma (CTCL) are diagnosed annually in the United States. This disease is characterized as a heterogeneous group of non-Hodgkin's lymphomas that manifest as T-lymphocyte infiltrates on the skin. The prognosis of this disease varies by severity, and treatment options include topical and systemic therapy. Vorinostat and romidepsin are histone deacetylase (HDAC) inhibitors that are approved for the treatment of CTCL. Although the mechanism of action of these agents is unknown, HDAC inhibitors exert their effects by interrupting various transcription factors, thereby producing cell-cycle arrest in some cancer lines. The purpose of this review is to evaluate the efficacy and safety of HDAC inhibitors for the treatment of CTCL.Cutaneous T-cell lymphoma (CTCL) is a heterogeneous group of non-Hodgkin's lymphomas (NHL) that are characterized by T lymphocytes that infiltrate the skin.1,2 There are 13 types of CTCL, as classified by the World Health Organization-European Organization for Research and Treatment of Cancer.3 The most common forms are mycosis fungoides (MF) and Sézary syndrome (SS).1,4 Patients with MF present with skin patches, plaques, tumors, and/or erythroderma. The prognosis of patients diagnosed with these types of CTCL is variable. Early-stage disease (e.g., MF) has a favorable prognosis; however, more aggressive lymphomas (e.g., SS) have poorer survival rates.1 MF is a slow-progressing malignancy that may worsen over years or even decades. SS, which is characterized by erythroderma, lymphadenopathy, and the presence of tumor cells in peripheral blood, is associated with a median survival of 32 months from diagnosis.5
Epidemiology
CTCL constitutes 3.4% of all cases of NHL.2 In the United States, approximately 1,100 new cases of CTCL are diagnosed annually.3 MF accounts for around 60% of new cases, and SS accounts for 5%. Black individuals have a twofold higher incidence of MF or SS than Caucasian individuals, and the disease is twofold higher in men than in women.5 Although the exact cause of CTCL has not been determined, possible etiologies include virus, inflammation, and immunosuppression.
Treatment
Treatment choices are governed by underlying disease severity, physician or patient preference, and institutional experience.5 Early-stage MF and SS respond to skin-directed therapies (SDTs) as monotherapy. Agents used for skin-directed therapy include topical corticosteroids, topical carmustine, bexarotene gel, ultraviolet B light, total-skin electron beam therapy, and superficial x-irradiation.5 Systemic therapies include chemotherapy, biological response modifiers (interferon, retinoids, denileukin diftitox), immunotherapy, extracorporeal photoimmunotherapy, and histone deacetylase (HDAC) inhibitors. Often, biologic therapy is combined with SDTs in patients with extensive or refractory skin disease or more advanced disease.5 The purpose of this review is to evaluate the efficacy and safety of the HDAC inhibitors vorinostat (Zolinza) and romidepsin (Istodax) for the treatment of CTCL.
Pharmacology
The precise mechanisms by which HDAC inhibitors exert their antitumor effects have not been elucidated. Histone proteins assemble genomic DNA in eukaryotic cells into a size and structure than can be accommodated by the nucleus. Histones are basic proteins that form the nucleosome. After the nucleosome is formed, an octamer wraps around the DNA. The nucleosome is condensed further by interacting with linker histones, which permit compaction and condensation.3
Two groups of enzymes regulate the acetylation of histone proteins. Histone acetyltransferases catalyze the transfer of acetyl groups on the side chain of lysine residues in target proteins. HDAC inhibitors counterbalance this activity through deacetylation of lysine residues. Although HDAC enzymes are classified as zinc-dependent (class I and II) or zinc-independent (class III), the majority of the enzymes in development are zinc-dependent.3 HDACs work by interacting with and repressing various transcription factors, and they have a number of different effects on the immune system (e.g., reducing acute graft-versus-host disease through suppression of proinflammatory cytokines, blocking of tumor cell lines).3 In vitro studies of romidepsin have revealed that accumulation of acetylated histones induces cell-cycle arrest and apoptosis of some cancer cell lines, with IC50 values in the nanomolar range.6 Vorinostat inhibits HDAC1, HDAC2, HDAC3 (class I), and HDAC6 (class II) at nanomolar concentrations. Like romidepsin, vorinostat is associated with reduced accumulation of acetylated histones and induces cell-cycle arrest and apoptosis of some cancer cell lines.7
Indications and Pharmacokinetics
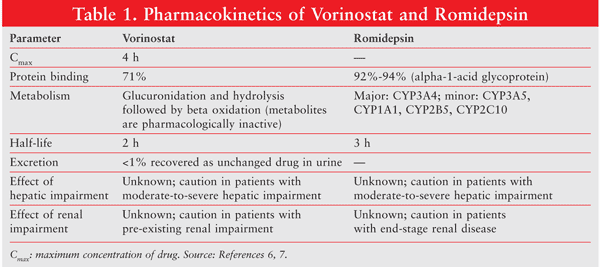
Romidepsin is indicated for the treatment of CTCL in patients who have received at least one prior systemic therapy.6 Vorinostat is indicated for the treatment of CTCL in patients who have progressive, persistent, or recurrent disease on or following two systemic therapies.7 The pharmacokinetics of the agents are similar.6,7 TABLE 1 provides pharmacokinetic information for vorinostat and romidepsin.6,7
Clinical Efficacy
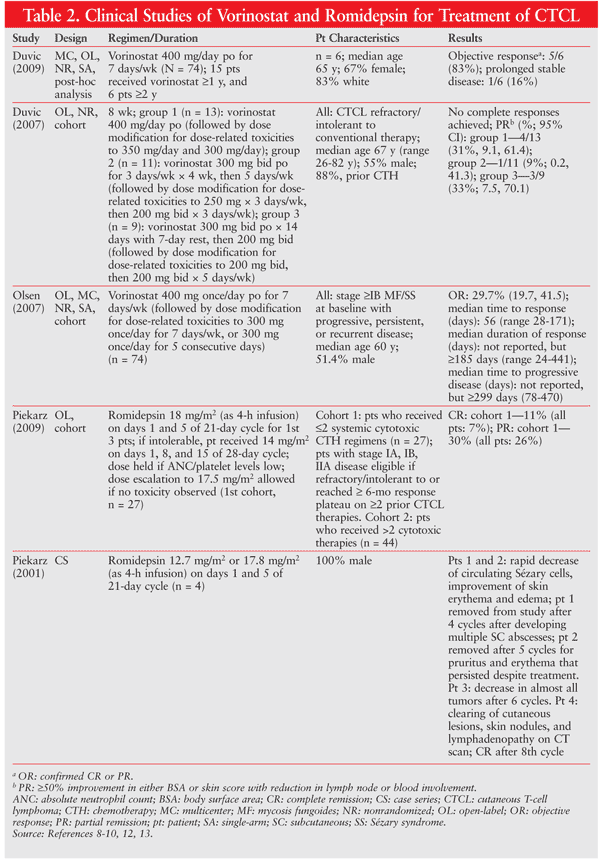
Several clinical studies have evaluated the efficacy of vorinostat and romidepsin in the treatment of CTCL. The trials were open-label and did not include comparison therapy. The majority of vorinostat-treated patients received vorinostat 400 mg orally daily. Dosage modifications were allowed for dose-related toxicities. Patients were refractory to conventional therapy or had progressive, persistent, or recurrent disease.8-10 The range achieved for partial response to therapy was 9% to 33% (average 30% in all patients; n = 74) in patients receiving vorinostat 400 mg daily, 300 mg twice daily for 7 days, and 300 mg twice daily for 14 days.9,11 Similarly, 29.7% of patients in another study achieved an objective response, and the median time to response was 55 days.10
Patients who received romidepsin had stage IA, IB, or IIA disease and were refractory or intolerant to other CTCL therapies.12 Romidepsin 18 mg/m2 was administered as a 4-hour infusion on days 1 and 5 of a 21-day cycle. The total proportion of patients who achieved a complete or a partial response, respectively, was 11% and 30%.12 A case series involving four patients revealed a rapid decline in circulating Sézary cells and improvement of skin erythema and edema in two patients. Decreases in tumor expression were observed in the other two patients with remission after 6 and 8 cycles, respectively.
The studies discussed above are summarized in TABLE 2.8-10,12,13
Adverse Effects
Vorinostat: Adverse effects associated with vorinostat therapy include diarrhea, fatigue, nausea, thrombocytopenia, anorexia, and dysgeusia. During the first 2 months of therapy, blood cell counts and chemistry tests (i.e., electrolytes, glucose, serum creatinine) should be performed every 2 weeks. Hypokalemia and hypomagnesemia should be corrected prior to vorinostat administration, and patients who present with symptoms of either of these conditions (i.e., nausea, vomiting, diarrhea, fluid imbalance, cardiac symptoms) should be monitored. Concomitant use with other HDAC inhibitors (e.g., valproic acid) has been associated with severe thrombocytopenia and gastrointestinal (GI) bleeding. In addition, prolongation of prothrombin time (PT) and international normalized ratio (INR) has been observed in patients receiving vorinostat concomitantly with warfarin anticoagulants. Careful monitoring of PT and INR is recommended in patients receiving vorinostat. Vorinostat therapy may be associated with QTc prolongation; therefore, patients who are predisposed to QTc prolongation should be carefully monitored while receiving vorinostat.6
Romidepsin: Adverse effects associated with romidepsin therapy include nausea, fatigue, infections, vomiting, anorexia, anemia, ECG T-wave changes, neutropenia, and lymphopenia.7 Therapy with romidepsin also may be associated with a potential for drug-drug interactions in patients who are receiving concomitant therapy with Coumadin derivatives. In addition, strong CYP3A4 inhibitors may increase concentrations of romidepsin, so their use should be avoided. Similarly, potent CYP3A4 inducers may reduce romidepsin concentrations. Hematologic parameters should be monitored in patients receiving romidepsin. Potassium and magnesium should be in the normal range in patients receiving romidepsin, as an increased risk of QT prolongation is associated with this therapy.7
Dosage and Formulations
Vorinostat is available in 100-mg capsules, and the recommended 400-mg dose should be administered once daily with food. Dosage reductions may be necessary in patients intolerant to therapy. The dosage may be reduced to 300 mg once daily with food for 5 consecutive days each week if intolerance to the initial dosage is observed. In clinical studies, adverse events necessitated dosage reductions in 10.5% (9/86) patients.7
Romidepsin is available as single-use vials containing 10 mg of romidepsin. Each kit also includes one sterile diluent vial containing 2 mL of 80% propylene glycol and 20% dehydrated alcohol. Romidepsin should be administered as 14 mg/m2 IV over a 4-hour period on days 1, 8, and 15 of a 28-day cycle. The cycle should be repeated every 28 days if clinical benefit and tolerability are achieved.6
Summary
Vorinostat and romidepsin are HDAC inhibitors used for the treatment of CTCL. The precise mechanism of action of these agents has not been determined; however, both drugs have been associated with accumulation of acetylated histones that are capable of inducing cell arrest and apoptosis in some cancer cells. Pharmacokinetic studies have revealed that romidepsin significantly impacts the CYP450 system and may induce several drug-drug interactions that do not occur with vorinostat therapy. Metabolism of vorinostat produces pharmacologically inactive metabolites. Clinical efficacy has revealed a response in up to 33% of treatment-experienced or -resistant patients in clinical studies. Fewer studies of romidepsin have been conducted; however, favorable results were observed. Several adverse drug reactions can occur with therapy, and additional monitoring is recommended for hematologic abnormalities, electrolyte disturbances, GI bleeding, and so on. Vorinostat is available in 100-mg capsules, and romidepsin is administered by IV infusion over 4 hours. Vorinostat and romidepsin may be beneficial for the treatment of CTCL, but additional studies are needed to define their role.
References
1. Gardner JM, Evans KG, Musiek A, et al. Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol. 2009;21:131-137.
2. Lansigan F, Choi J, Foss FM. Cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:979-996.
3. Khan O, La Thangue NB. Drug insight: histone deacetylase inhibitor-based therapies for cutaneous T-cell lymphomas. Nat Clin Pract Oncol. 2008;5:714-726.
4. Horwitz SM. T-cell lymphomas. Clin Adv Hematol Oncol. 2009;7:380-382.
5. Trautinger F, Knobler R, Willemze R, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer. 2006;42:1014-1030.
6. Istodax (romidepsin) package insert. Cambridge, MA: Gloucester Pharmaceuticals; November 2009.
7. Zolinza (vorinostat) package insert. Whitehouse Station, NJ: Merck; February 2010.
8. Duvic M, Olsen EA, Breneman D, et al. Evaluation of the long-term tolerability and clinical benefit of vorinostat in patients with advanced cutaneous T-cell lymphoma. Clin Lymphoma Myeloma. 2009;9:412-416.
9. Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109:31-39.
10. Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109-3115.
11. Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318-2322.
12. Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410-5417.
13. Piekarz RL, Robey R, Sandor V, et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865-2868.
To comment on this article, contact rdavidson@uspharmacist.com.





