US Pharm. 2011;36(7)(Oncology suppl):12-15.
ABSTRACT: Oral mucositis is a widespread and potentially serious consequence of high-dose chemotherapy and radiotherapy. It seems to be particularly associated with fluorouracil, doxorubicin, and methotrexate. Symptoms, which may include altered taste perception, sores, and varying degrees of pain, usually appear 4 to 5 days after treatment initiation. Treatment is mainly supportive, involving both nonpharmacologic and pharmacologic methods. For compounded preparations such as mouthwashes, there are various formulations that pharmacists can use based on the experience and needs of the individual physician and patient, respectively.
Mucositis, a painful inflammation and ulceration of the mucous membranes lining the digestive tract, can involve both the oral tract and the gastrointestinal tract. Oral mucositis is a widespread and potentially serious consequence of high-dose chemotherapy and radiotherapy treatments that appears to be particularly associated with fluorouracil, doxorubicin, and methotrexate.1 It is a devastating consequence of cancer treatment for patients, who find it extremely difficult to perform simple, everyday mouth-related activities, including chewing, biting, talking, swallowing, drinking, and sipping.
Oral and gastrointestinal mucositis affects up to 100% of patients undergoing high-dose chemotherapy and hematopoietic stem-cell transplantation and 80% of patients with malignancies of the head and neck who are receiving radiotherapy. Mucositis also affects a wide range of patients receiving chemotherapy.
Causes of Mucositis
The development of mucositis involves five stages: (1) initiation, (2) message generation, (3) signaling and amplification, (4) ulceration, and (5) healing. Different cytokines are responsible for the various stages (TABLE 1).2 Initiation results from the production of free radicals caused by chemotherapy or radiotherapy, which damages cell DNA. This results in the production of cell transcription factors that upregulate inflammatory cytokines, marking the beginning of ulceration. During the healing stage, epithelial cells are attracted to the site of the ulcer and begin re-epithelialization.2
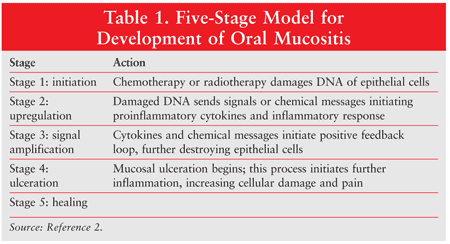
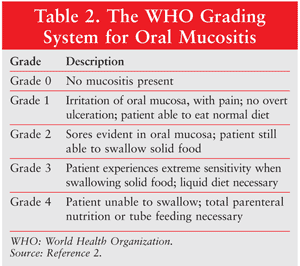
About 5% to 15% of all patients undergoing cancer treatment experience mucositis. TABLE 2 details the grades of oral mucositis as defined by the World Health Organization (WHO).2,3 In grade 3 oral mucositis, the patient is unable to eat solid food; in grade 4, the patient is also unable to consume liquids. Radiotherapy to the head and neck or to the pelvis or abdomen is associated with grade 3 oral mucositis in more than 50% of patients. Among patients undergoing head and neck radiotherapy, pain and decreased oral function may persist long after the conclusion of therapy. HIV infection may cause oral mucocutaneous candidiasis.
Oral mucositis is characterized by damage to the epithelium of the oropharyngeal cavity. Because of their relatively high turnover rate compared with cells in other organs, epithelial cells are particularly susceptible to the damaging effects of radiation and chemotherapy. A reduction of the saliva barrier, disruption of epithelial cells, thinning of the epithelium, and ulceration also occur. Preexisting conditions can contribute to oral mucositis, including oral infections and trauma or irritation; the patient’s gender and age also may be involved.
Symptoms
Cancer patients who are undergoing chemotherapy usually become symptomatic 4 to 5 days after treatment is initiated. A peak is reached at around day 10, followed by slow improvement over the course of a few weeks. Radiotherapy-induced mucositis, which usually appears at the end of the second week of treatment, may last for 6 to 8 weeks.
As a result of epithelial-cell death, the mucosal lining of the mouth thins, sloughs off, and becomes red, inflamed, and ulcerated; peripheral erythema is usually present. Ulcers range in size from 0.5 cm to greater than 4 cm. Oral mucositis can be extremely painful; the degree of pain, often described as a burning sensation, is usually related to the extent of the tissue damage. As a result of the pain, the patient may experience trouble speaking, eating, or even opening the mouth.
Alterations in taste perception are common, especially in patients receiving concomitant radiation therapy to the neck and mouth. An altered sense of taste is a temporary condition that occurs because of effects on the taste buds located mainly in the tongue. Common complaints are that food tastes too sweet or too bitter or continually has a metallic taste. In some cases, only partial recovery of taste occurs. Severe pain and the inability to chew, swallow, or talk may ultimately lead to dehydration, malnutrition, anorexia, and cachexia. The lack of saliva can cause tooth caries, loss of fillings, mouth ulcers, and pain, as saliva has a cleansing effect on the mouth and controls bacteria.3
Pain and loss of taste perception make it more difficult to eat, which frequently leads to weight loss. Infection of the sores or ulcerations by a virus, bacterium, or fungus can occur, and in some instances may cause septicemia (especially in immunosuppressed patients). About half of all patients receiving chemotherapy develop severe oral mucositis that becomes dose-limiting, requiring modification of the patient’s cancer treatment.
Diagnosis
The diagnosis of oral mucositis is based on the symptoms the patient is experiencing and the appearance of the tissues in the mouth following chemotherapy or radiotherapy. The severity of oral mucositis can be evaluated according to several different assessment tools (TABLES 2 and 3).2,4 A number of scales are available, including those developed by the WHO, the Radiation Therapy Oncology Group, the Western Consortium for Cancer Nursing Research, and the National Cancer Institute (NCI), among others.4
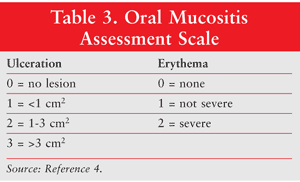
Two of the most commonly used scales are the WHO Oral Toxicity Scale and the NCI Common Toxicity Criteria for oral mucositis. While the NCI system has separate scores for appearance (erythema and ulceration) and function (pain and ability to eat solids, liquids, or nothing by mouth), the WHO score combines both elements into a single score that grades the severity of the condition from 0 (no oral mucositis) to 4 (swallowing not possible, such that patient needs supplementary nutrition), as described in TABLE 2.2 Another scale, the Oral Mucositis Assessment Scale (OMAS), was developed in 1999. It has been shown to be highly reproducible between observers, responsive over time, and accurate in recording symptoms associated with mucositis. The OMAS provides an objective assessment of oral mucositis based on an evaluation of the appearance and the extent of redness and ulceration in various areas of the mouth.
Treatment
Treatment of mucositis is mainly supportive, with both nonpharmacologic and pharmacologic options.5 Examples of nonpharmacologic methods include:
1. Preventive strategies involving a pretreatment dental examination and improved dental hygiene, since good oral hygiene is the mainstay of treatment. Patients should be encouraged to clean the mouth every 4 hours and at bedtime, and more often if the mucositis worsens. Patients should have the mouth checked regularly, brush the teeth with a gentle toothbrush two or three times daily, use a nondetergent toothpaste, floss between the teeth, and use an alcohol-free mouthwash.
2. The use of artificial saliva and water-soluble jellies to lubricate the mouth. Dry-mouth lozenges and dry-mouth gum also may be used.
3. The use of saline or baking soda mouthwash to soothe the pain and remove food particles to avoid infection.
4. Drinking plenty of liquids—at least 3 liters per day—and eschewing alcohol.
5. Avoiding citrus fruits, tomatoes, acidic foods, alcohol, and hot foods that can aggravate mucositis lesions. Ingested food should be soft, nonspicy, pureed, or in liquid form. Harder foods should be treated to make them soft and easy to eat.
6. Refraining from smoking.
7. Sucking ice cubes. This is a simple, but often effective, method of relieving the symptoms of oral mucositis.
Examples of pharmacotherapy include5,6:
1. Medicinal mouthwashes, such as chlorhexidine gluconate and viscous lidocaine, for pain relief.
2. Palifermin, a human keratinocyte growth factor that enhances epithelial cell proliferation, differentiation, and migration.
3. Barrier-protection agents, such as concentrated oral gel products (e.g., Gelclair, MuGard).
4. Caphosol, a mouth rinse for preventing and treating oral mucositis.
5. Analgesics (e.g., capsaicin) and sedatives for pain reduction.
6. Multipurpose mouthwashes (Swish and Swallow, Swish and Spit). Routinely prepared by compounding pharmacists, these mouthwashes are commonly prescribed and come in a multitude of formulations. Their composition varies, but may include combinations of antihistamines (chlorpheniramine, diphenhydramine), antibiotics (tetracycline), anti-inflammatories, antifungals (nystatin, miconazole), corticosteroids (hydrocortisone, clobetasol), anesthetics (lidocaine, dicyclonine) and other agents, such as silver nitrate, misoprostol, leucovorin, pentoxifylline, allopurinol, chlorhexidine gluconate, acyclovir, valacyclovir, or beta-carotene tocopherol.4
7. Numerous dosage forms of the drugs listed above, such as troches, lollipops, popsicles, pastes, and rinses. These can be compounded for individual patients to meet their needs for ease of administration.
Compounded Preparations
Pharmacists can make an invaluable contribution by counseling patients and encouraging them to share what they are experiencing. Pharmacists can assist in modifying the compounded preparations to help the patient be more compliant.
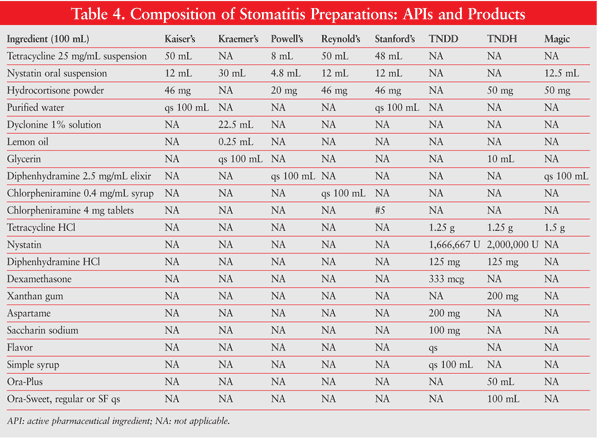
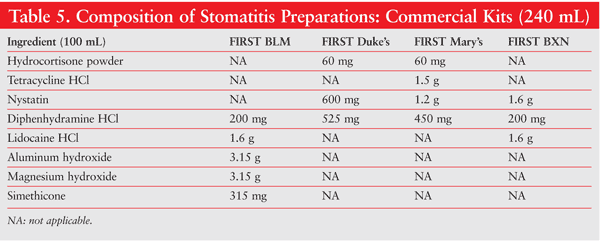
Numerous formulations are possible, depending upon what the individual physician requires. Different drugs, concentrations, flavors, sweeteners, viscosities, and so on, may be considered, based on the patient’s needs and preferences. Several widely used oral mouthwash formulations for oral mucositis are Kaiser’s, Kraemer’s, Powell’s, Reynold’s, Stanford’s, TNDD (tetracycline, nystatin, diphenhydramine, dexamethasone), and TNDH (tetracycline, nystatin, diphenhydramine, hydrocortisone) (TABLE 4). Optionally, commercial kits designed to make the compounding of oral mucositis preparations easier and more convenient may be used. Available kits include Mouthwash BLM (diphenhydramine, lidocaine, magnesium and aluminum hydroxides), Duke’s Mouthwash (diphenhydramine, hydrocortisone, nystatin), Mary’s Mouthwash (diphenhydramine, hydrocortisone, nystatin, tetracycline), and BXN Mouthwash (diphenhydramine, lidocaine, nystatin), which are all alcohol free (TABLE 5).
The compounding of oral liquid mouthwash formulations may be accomplished by using commercial drug products (diphenhydramine elixir) or bulk chemicals or active pharmaceutical ingredients (diphenhydramine hydrochloride). When commercial products containing alcohol are used, it is generally best to mix the products in a manner that keeps the alcohol concentration as high as possible during the compounding procedure. This helps maintain alcohol-soluble ingredients in solution. Bulk powders that are highly water soluble generally do not require any pretreatment; if the powders are poorly water soluble, however, it is advisable to reduce their particle size prior to incorporation into the product. If capsules or tablets are used, the particle size of the capsule contents or tablets should be reduced to a very fine state of subdivision. In working with viscous preparations, it is best to add the viscous substances together slowly, while mixing, to minimize problems.
References
1. Medina PJ, Fausel C. Cancer treatment and chemotherapy. In: DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill Medical; 2008:2115-2116.
2. Maxon J. Advances in the prevention and management of oral mucositis. Current Topics in Oncology.
http://professional.
3. Silverman S Jr. Diagnosis and management of oral mucositis. J Support Oncol. 2007;5(2 suppl1):13-21.
4. Kepivance. Assessment of oral mucositis.
www.kepivance.com/oral_
5. Hsaio G, Sonis S. Oral mucositis. In: Interactive Textbook on Clinical Symptom Research.
http://painconsortium.nih.gov/
6. Cornett PA, Dea TO. Cancer. In: McPhee SJ, Papadakis MA, Rabow MW, eds. Current Medical Diagnosis and Treatment 2011. 50th ed. New York, NY: McGraw-Hill Medical; 2011:1591.
To comment on this article, contact rdavidson@uspharmacist.com.





