US Pharm. 2012;37(7)(Oncology suppl):8-11.
ABSTRACT: Renal cell carcinoma (RCC), the most common form of kidney cancer in the United States, affects mainly older adults and is more likely to occur in men. Genetic, lifestyle, and environmental factors are all contributors to the development of this disease. Most patients are asymptomatic when they are diagnosed with RCC, which may already be locally advanced or metastatic. Treatment options include surgery (the mainstay of treatment), immunotherapy, chemotherapy, and targeted therapy. Metastatic RCC remains incurable, although the expanded arsenal of treatment options has improved survival.
Renal cell carcinoma (RCC) is the most common type of kidney cancer in the United States. The American Cancer Society estimated that 60,920 new cases would be diagnosed in 2011, with 13,120 people dying from the disease.1 The incidence of RCC has gradually risen since 1975, partly because of the increased performance of radiographic imaging for unrelated health conditions. RCC mostly affects older adults, and men are at higher risk. RCC is rare in children and adults younger than 50 years of age. Many renal carcinomas are clinically silent until metastasis has occurred.
RCC is an immunologic malignancy that impairs tumor immunity by indirectly affecting the proliferation of regulatory T cells and myeloid-derived suppressor cells, thus activating a proinflammatory state in T-helper cells.2 Most RCCs are classified as clear cell carcinoma, but they can also be papillary carcinoma, chromophobe RCC, or collecting duct carcinoma. In general, the prognosis for advanced RCC is poor.
Risk Factors and Molecular Pathogenesis
Environmental, lifestyle, and genetic factors all play a role in the development of RCC. Smoking, hypertension, and obesity have been linked to RCC, with smoking increasing the risk by 30% to 100% and leading to poorer survival outcomes in RCC patients.3 Asbestos, cadmium, and trichloroethylene exposure are associated with RCC. Predisposing conditions such as long-term dialysis and hereditary syndromes contribute to the development of RCC. Acquired cystic kidney disease associated with end-stage renal disease has been linked to the development of RCC. Long-term dialysis patients are three to six times more likely than healthy individuals to develop RCC.
Von Hippel-Lindau disease (VHL) is an autosomal-dominant hereditary condition caused by inactivation of the VHL tumor-suppressor gene located on chromosome 3. This gene mutation is related to the development of RCC.4 The VHL gene normally inhibits hypoxia-inducible factor (HIF), which is the major homeostasis regulator of hypoxia-responsive genes such as epidermal growth factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and other proangiogenic factors.4 Mutation of the VHL gene causes an accumulation of HIF, which promotes abnormal formation of blood vessels that can lead to tumor development in patients with VHL.
Other hereditary syndromes that may predispose an individual to RCC are the Birt-Hogg-Dubé (BHD) syndrome and the hereditary leiomyomatosis and RCC syndrome (HLRCC). BHD, an autosomal-dominant condition associated with renal cancer, is caused by germline mutations in the FLCN gene, the encoder for folliculin, and is associated with the mammalian target of rapamycin (mTOR) pathway.5 mTOR stimulates the production of HIF and VEGF.6 HLRCC is an autosomal-dominant disorder caused by a mutation in the FH gene. Renal cell cancers develop in up to 16% of patients with HLRCC.
RCC development also can be caused by gene abnormalities that regulate cell division, including the ras family genes and the p53 tumor-suppressor gene.7
Clinical Presentation
Most patients with RCC are asymptomatic at the time of diagnosis. The most common symptoms of RCC include the classic triad of hematuria, flank pain, and abdominal mass, although few patients present with this triad. Other symptoms are fatigue, fever, weight loss, and laboratory abnormalities such as anemia, abnormal liver function, and hypercalcemia. At diagnosis, most patients already have locally advanced or metastatic disease. The most common sites to which RCC can metastasize include the lungs, lymph nodes, bone, liver, pancreas, and retroperitoneum. Over the past several years, radiographic imaging (e.g., CT and MRI) has incidentally diagnosed RCC, since these methods are widely used to evaluate unrelated problems.3,8
Diagnosis
There is no defined diagnostic protocol for RCC, but if RCC is suspected, the initial workup includes several diagnostic tests in addition to the patient’s history and physical examination. The National Comprehensive Cancer Network (NCCN) 2012 guidelines for kidney cancer recommend a CBC including a lactic dehydrogenase level, urinalysis, and abdominal and/or pelvic CT or MRI with or without contrast.9 If a suspicious mass is present, the workup should include chest imaging, a bone scan or brain MRI if clinically indicated, and urine cytology or ureteroscopy if urothelial carcinoma is suspected.9 Small lesions may be biopsied to confirm a diagnosis of malignancy and inform treatment strategies.9 Based on these findings, treatment can be determined once the disease is staged.
Staging
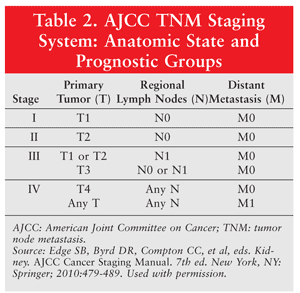
Management of RCC depends upon the stage of the disease according to the tumor node metastasis (TNM) staging system (TABLES 1 and 2). The TNM staging system is based upon the size of the primary tumor, regional lymph node involvement, and metastasis. As the disease stage increases, the survival rate decreases. In stage I, the tumor is less than 7 cm and is confined to the kidney (no lymph node involvement or distant metastasis). Stage II differs from stage I in the size of the tumor present in the kidney (7-10 cm). Stage III involves regional lymph node metastasis and tumor extension to the veins, tissues, and/or vena cava. In stage IV, the tumor extends beyond Gerota’s fascia, with or without lymph node involvement or distant metastasis.9

Treatment Options
Surgery: Surgical resection, a curative therapeutic option, is the mainstay of treatment for patients with localized disease. This approach is determined by the size and location of the tumor, the staging system, evaluation for metastases, and the involvement of other organs. Radical nephrectomy is the removal of the kidney, Gerota’s fascia, ureter, ipsilateral adrenal gland, and adjacent hilar lymph nodes.10 Partial nephrectomy (also known as nephron-sparing surgery) or radical nephrectomy is indicated for stage I disease. Radical nephrectomy is indicated in stages II and III, and is preferred when the tumor extends into the inferior vena cava.9 Patients without metastatic disease are most likely to benefit from surgery. In patients with nonmetastatic disease, 5-year survival is 40% to 65%, compared with 7% to 28% in patients with metastatic disease.11
Radiofrequency ablation (RFA) and cryotherapy are alternative surgical options for patients in whom the traditional partial or radical nephrectomy is not appropriate. RFA emits high-energy radio waves that are passed through a thin needle directly into the tumor.12 In cryotherapy, a cold metal probe is inserted into the tumor, which freezes the tumor and causes its death.
Immunotherapy: Before the introduction of targeted agents, immunotherapy was the primary treatment modality for metastatic RCC (mRCC). Interferon (IFN) and interleukin-2 (IL-2) were used for patients with metastatic, recurrent, or unresectable clear cell RCC. IL-2 and IFN have been shown to produce a response in selected patients, with a modest effect on survival. In clinical trials, high-dose IL-2 yielded durable complete responses in 7% to 28% of patients, with a median duration of approximately 19 months; its use is warranted in patients with low-volume disease, good performance status, and predominantly clear cell carcinoma.2,9 Unfortunately, high-dose IL-2 has a toxicity profile that limits its use. It is known to cause significant dose-related morbidity, although mortality rates have decreased with its use. The most common serious adverse effect with IL-2 is capillary leak syndrome, which results in a hypovolemic state and fluid accumulation in the extravascular space and can lead to oliguria, ischemia, and confusion.13 Other toxicities involve the heart, lungs, kidneys, and central nervous system. Despite its adverse effects, IL-2 can greatly benefit a small number of patients.
IFN, the first cytokine to show activity against advanced RCC, historically was the standard front-line treatment. However, as a single agent, IFN demonstrated a clinical benefit of a median 13-month survival in only a small number of patients.2 In the global AVOREN trials, IFN in combination with bevacizumab (a recombinant humanized monoclonal antibody that binds and neutralizes circulating VEGF-A) demonstrated a greater progression-free survival (PFS)—10.2 versus 5.4 months—and an objective tumor response rate of 30.6% versus 12.4%.9,14 The NCCN guidelines for kidney cancer recommend this combination as first-line treatment for predominantly clear cell carcinoma. The adverse-effect profile for these agents combined is tolerable overall. Patients receiving bevacizumab should be monitored for hypertension, proteinuria, and epistaxis. IFN can cause flulike symptoms within a couple of hours of administration that can last up to 24 hours.
Vaccines: Vaccines for the prevention or treatment of RCC are currently being investigated, but they have yet to show survival advantages in randomized phase III trials.15
Chemotherapy: Chemotherapy generally is not effective for RCC and has not been demonstrated to improve survival in mRCC. However, in combination with other therapies, gemcitabine has shown some activity against mRCC. The combination of gemcitabine and doxorubicin is recommended by the NCCN as first-line treatment for patients with relapsed or medically unresectable stage IV disease with non–clear cell histology.9
Targeted Therapy
Because of the growing understanding of the biological changes occurring in RCC, agents that target the VEGF pathway have been investigated, leading to multiple new treatment options for mRCC in the past several years. VEGF receptors are involved in pathologic angiogenesis, tumor growth, and cancer progression. Targeted therapies that are approved for stage IV mRCC include sunitinib, sorafenib, pazopanib, axitinib, temsirolimus, everolimus, and bevacizumab in combination with IFN (TABLE 3).9
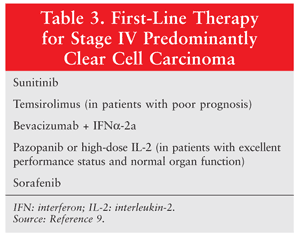
Sunitinib, sorafenib, pazopanib, and axitinib are tyrosine kinase inhibitors (TKIs). Tyrosine kinases (TKs) play a vital role in cell signaling and modulation of growth factors. TKIs are targeted therapies that inhibit tumor cell proliferation and angiogenesis in RCC. Sunitinib, the current standard of care for mRCC, works by inhibiting multiple receptor TKs, including VEGF, PDGF, and colony-stimulating factor type 1. A randomized phase III trial demonstrated improved PFS for sunitinib versus IFN-alfa (11 months vs. 5 months, respectively) as first-line treatment for mRCC; it also improved overall survival.16 The dosage of sunitinib is 50 mg orally once daily for 4 weeks, with 2 weeks off.
Sorafenib was the first TKI to be approved for mRCC (in December 2005). It works by inhibiting several TKs and is used in patients who have previously failed standard treatment for advanced RCC.17 The dosage of sorafenib is 400 mg orally twice daily.
In October 2009, the FDA approved pazopanib for the treatment of advanced mRCC. In a phase III, placebo-controlled, randomized, double-blind, multicenter study, pazopanib achieved significantly prolonged PFS compared with placebo (9.2 months vs. 4.2 months).18 The dosage of pazopanib is 800 mg orally once daily.
In January 2012, axitinib became the most recent drug to be approved for the treatment of mRCC after failure of a prior VEGF inhibitor or cytokine. Axitinib more selectively inhibits the VEGF pathway and has fewer off-target effects than the earlier TKIs.19 The dosage of axitinib is 5 mg orally twice daily. Other TKIs currently undergoing clinical trials include tivozanib and cediranib.
Because the TKIs currently used to treat RCC are multitargeted agents that inhibit a number of receptor kinases, numerous adverse effects can occur. Common effects include cardiovascular events, hypothyroidism, hand-foot syndrome, diarrhea, stomatitis, fatigue, and hypertension.20
Temsirolimus is the first mTOR inhibitor for the treatment of advanced RCC.21 Its active metabolite, sirolimus, binds to an intracellular protein, stopping mTOR signaling and the cell cycle at G1 phase in the tumor cells; it also decreases VEGF and HIF. Temsirolimus is approved as first-line therapy in patients with advanced RCC who have a poor prognosis. Common reactions to temsirolimus are rash, peripheral edema, hyperglycemia, and hyperlipidemia.22 The dosage of temsirolimus is 25 mg IV weekly until disease progression or occurrence of toxicity.
Everolimus is an oral mTOR inhibitor that is FDA-approved for second-line therapy after failure of VEGF-targeted therapies (sorafenib or sunitinib). Approval was based on a 2008 phase III study in which PFS was 4.9 months for everolimus vs. 1.9 months for placebo.23 The dosage for advanced RCC is 10 mg orally once daily. Common adverse effects include fatigue, stomatitis, rash, infections, hyperglycemia, hypertriglyceridemia, and pneumonitis.24
Conclusion
The past decade has provided clinicians with several new treatment options for mRCC in the form of the VEGF and mTOR inhibitors. The expansion of treatment options evidently has improved patient survival, as well as PFS. However, mRCC remains an incurable disease. Surgery is the foundation of treatment for patients with stage I, II, or III disease, but more clinical trials are needed to investigate evolving treatments, such as vaccines, as well as combination therapy with targeted agents for all stages of RCC.
REFERENCES
1. American Cancer Society. Kidney cancer (adult)—renal cell
carcinoma.
http://documents.cancer.org/acs/groups/cid/documents/webcontent/003107-pdf.pdf.
Accessed May 9, 2012.
2. George S, Pili R, Carducci MA, Kim JJ. Role of immunotherapy for renal cell cancer in 2011. J Natl Compr Canc Netw. 2011;9:1011-1018.
3. Chabner BA, Lynch TJ Jr, Longo DL. Harrison’s Manual of Oncology. New York, NY: McGraw-Hill Professional; 2008:345.
4. Arjumand W, Sultana S. Role of VHL gene mutation in human renal cell carcinoma. Tumour Biol. 2012;33:9-16.
5. Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199-1206.
6. Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45.
7. Atkins MB, Choueiri TK. Epidemiology, pathology, and pathogenesis
of renal cell carcinoma. UpToDate.
www.uptodate.com/contents/epidemiology-pathology-and-pathogenesis-of-renal-cell-carcinoma.
Accessed May 3, 2012.
8. Skeel RT, Khleif SN. Handbook of Cancer Chemotherapy. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:250-257.
9. National Comprehensive Cancer Network. NCCN Clinical Practice
Guidelines in Oncology. Kidney cancer. Version 1.2012.
www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed May 3,
2012.
10. Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477-2490.
11. Torrey R, Spiess PE, Pal SK, Josephson D. Role of surgery for locally advanced and metastatic renal cell carcinoma. J Natl Compr Canc Netw. 2011;9:985-993.
12. Kwan KG, Matsumoto ED. Radiofrequency ablation and cryoablation of renal tumours. Curr Oncol. 2007;14:34-38.
13. Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park). 2002;16(11 suppl 13):11-20.
14. Harshman LC, Srinivas S. The bevacizumab experience in advanced renal cell carcinoma. Onco Targets Ther. 2010;3:179-189.
15. Kabaker K, Shell K, Kaufman HL. Vaccines for colorectal cancer and renal cell carcinoma. Cancer J. 2011;17:283-293.
16. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and
updated results for sunitinib compared with interferon alfa in patients
with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
17. Jonasch E, Hutson TE, Harshman LC, Srinivas S. Advanced renal
cell carcinoma: overview of drug therapy for the practicing physician.
In: ASCO 2011 Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2011:145-151.
18. Vasudev NS, Larkin JM. Tyrosine kinase inhibitors in the treatment of advanced renal cell carcinoma: focus on pazopanib. Clin Med Insights Oncol. 2011;5:333-342.
19. CancerNetwork. Advances and new research in the treatment of
kidney cancer [podcast].
www.cancernetwork.com/kidney-cancer/content/article/10165/2026106.
Accessed February 6, 2012.
20. Eisen T, Sternberg CN, Robert C, et al. Targeted therapies for
renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104:93-113.
21. Torisel (temsirolimus) product information. Philadelphia, PA: Wyeth Pharmaceuticals, Inc; January 2012.
22. Bajaj M, Heath EI. Targeted therapy for the treatment of renal cell carcinoma. In: ASCO 2011 Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2011:e4-e7.
23. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in
advanced renal cell carcinoma: a double-blind, randomised,
placebo-controlled phase III trial. Lancet. 2008;372:449-456.
24. Afinitor (everolimus) product information. East Hanover, NJ: Novartis Pharmaceuticals Corp; April 2012.
To comment on this article, contact rdavidson@uspharmacist.com.