US Pharm. 2013;38(7)(Oncology suppl):8-11.
ABSTRACT: The mortality rate for ovarian cancer, the second most common gynecologic malignancy, has not changed appreciably in the last 40 years. Often, the diagnosis of this disease is delayed because the symptoms are vague and nonspecific. Surgery and platinum-based chemotherapy are the primary treatment modalities for ovarian cancer. The prognosis is favorable for early-stage disease, but approximately 60% of cases are metastatic upon presentation. Many novel targeted therapies are under investigation in hopes of improving outcomes in patients diagnosed with ovarian cancer.
Ovarian cancer is the second most common gynecologic malignancy, with an estimated 22,280 new cases in 2012. An estimated 15,500 deaths were attributed to ovarian cancer in 2012, making it the deadliest gynecologic malignancy.1 Despite improvements in the treatment of ovarian cancer, mortality has remained largely unchanged over the last 40 years.2 Ovarian cancer has been called the “silent killer” because the symptoms are vague and nonspecific, leading to delayed diagnosis. While the prognosis is good for early-stage disease, approximately 60% of patients present with metastatic disease.2
Surgery and platinum-based chemotherapy are the primary treatment modalities for ovarian cancer. This review focuses on the management of epithelial ovarian cancer, which constitutes more than 80% of ovarian cancers.3 Current standards of care and the use of intraperitoneal (IP) chemotherapy will be discussed, and a brief overview of new agents being studied for their utility in improving treatment outcomes will be presented.
RISK FACTORS
A number of factors affect the risk of ovarian cancer (TABLE 1). As with most cancers, the risk of ovarian cancer rises with increasing age; the patient’s age at diagnosis is in the early 60s, on average.4 Additionally, the incessant-ovulation hypothesis claims that the risk increases in accordance with the number of ovulations because of the repair required each time ovulation occurs.5 Therefore, conditions that increase a woman’s total number of ovulations—including early menarche, nulliparity, and late menopause—are associated with a higher risk of ovarian cancer. Recent cohort studies have found that hormone replacement therapy and pelvic inflammatory disease (PID) may also increase risk.6,7 Conversely, multiparity, the use of oral contraceptives, and breastfeeding may reduce the risk of ovarian cancer.5
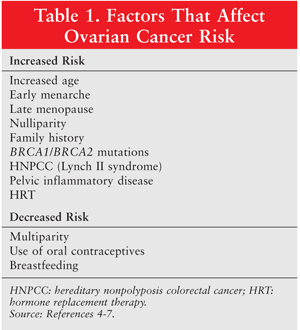
Family history and genetic mutations also contribute to the development of ovarian cancer. The risk increases with the number of first- and second-degree relatives diagnosed with ovarian cancer, and hereditary cases are often linked to the breast cancer susceptibility genes BRCA1 and BRCA2.5 Hereditary nonpolyposis colorectal cancer, also known as Lynch II syndrome, increases the risk of ovarian cancer, colorectal cancer, and other malignancies.5
CLINICAL PRESENTATION
Signs and symptoms suggestive of ovarian cancer are vague and nonspecific, with early stages often presenting asymptomatically. Symptoms such as bloating, pelvic/abdominal pain, nausea, urinary urgency or increased frequency, and difficulty eating or early satiety may be confused with menopause, depression, or gastrointestinal disorders.4 Signs that may help distinguish ovarian cancer from other possible diseases include vaginal bleeding (which may be irregular), ascites, elevated cancer antigen 125 (CA-125), and palpable pelvic/abdominal masses. These signs are more likely in advanced stages of ovarian cancer, resulting in delayed diagnosis for many patients.
DIAGNOSIS AND STAGING
Patients presenting with the above symptoms or a palpable pelvic mass should undergo a thorough examination. Approximately 90% of patients with malignant ovarian tumors present with elevated CA-125.4 Elevated CA-125 is not diagnostic of ovarian cancer, since it may also occur in common benign conditions such as endometriosis and PID. However, it is helpful for monitoring patient response to treatment and for identifying recurrent disease in patients who have previously responded to therapy.
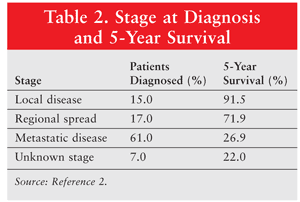
Various imaging modalities, such as transvaginal ultrasound (TVUS) and CT scan, may be used to identify and further evaluate ovarian tumors.8 TVUS is an imaging test used to differentiate benign tumors from malignant cancers, and CT scans are often performed to detect advanced or metastatic disease. Although these imaging tests are helpful for the initial workup, radiographic imaging and needle biopsies typically are insufficient for diagnosis. Most patients with suspicious ovarian masses will undergo surgery for a definitive diagnosis and surgical staging. Staging of ovarian cancer follows the primary tumor, regional lymph nodes, and distant metastasis (TNM) and International Federation of Gynecology and Obstetrics (FIGO) system, with tumors classified as stages I to IV. The distribution of stage at diagnosis and correlating 5-year survival rates are listed in TABLE 2.
TREATMENT
Surgery
The initial treatment for nearly all patients is surgery for staging and tumor removal, even in patients with metastatic disease. Most patients require extensive tumor debulking consisting of total abdominal hysterectomy, bilateral salpingo-oophorectomy, and possible removal of other pelvic and abdominal structures required for complete tumor dissection.8 Rarely, conservative surgery may be used to maintain fertility if a patient is young and has localized disease. The selection of a surgeon is important, since patient survival is improved when the initial surgical management of ovarian cancer is performed by a gynecologic oncologist.9
Chemotherapy
Platinum-based chemotherapy is administered to nearly all ovarian cancer patients. Data are conflicting regarding whether chemotherapy should be administered prior to surgery.10-13 According to a survey of members of the Society of Gynecologic Oncologists, the majority of gynecologic oncologists rarely utilize neoadjuvant chemotherapy and prefer to perform initial tumor debulking surgery prior to administering chemotherapy.14 However, recent data support the use of neoadjuvant chemotherapy in selected patients with bulky stage III or IV ovarian cancer who are not surgical candidates at the time of diagnosis.11,12,15
With the exception of patients with stage IA or IB tumors, in which observation after surgery is acceptable, patients should receive adjuvant chemotherapy following primary cytoreductive surgery.16 First-line treatment options are given in TABLE 3.
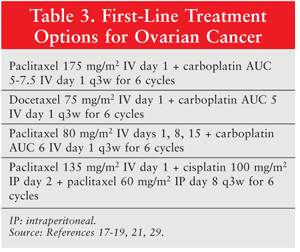
The Gynecologic Oncology Group study 158 (GOG 158) demonstrated that carboplatin plus paclitaxel was noninferior to, and better tolerated than, cisplatin plus paclitaxel; this became the standard adjuvant regimen.17
The Scottish Gynecological Cancer Trials Group conducted a clinical trial comparing carboplatin plus paclitaxel or docetaxel.18 Carboplatin plus docetaxel was noninferior to carboplatin plus paclitaxel, and this combination may be considered an alternative first-line regimen. Docetaxel patients experienced more grade 3 or 4 neutropenia, whereas paclitaxel patients exhibited more neurotoxicity; therefore, regimen selection may be based on adverse-effect profiles and patient characteristics.
Carboplatin with dose-dense paclitaxel may be considered, based on results of a clinical trial conducted by the Japanese Gynecologic Oncology Group.19 Carboplatin plus paclitaxel administered every 3 weeks (as studied in GOG 158) was compared with carboplatin plus weekly paclitaxel. Median progression-free survival (PFS) significantly improved in the dose-dense arm (28 months vs. 17.2 months for controls), as did overall survival (OS) at 3 years (72.1% vs. 65.1%). However, the dose-dense arm exhibited significantly more grade 3 or 4 anemia, and withdrawal due to toxicity occurred more frequently in this arm than in controls. Carboplatin with weekly paclitaxel is therefore considered a first-line option, but clinicians must remember that not all patients can tolerate this regimen.
IP chemotherapy is a unique method that may be considered for eligible stage II or III patients. An abdominal port is placed, typically during the initial tumor debulking surgery, to deliver drug directly into the peritoneal cavity. This mode of administration allows significantly greater concentrations of the chemotherapeutic agent to be delivered directly to the tumor site.20 In the GOG 172 clinical trial, combination IP and IV chemotherapy improved survival versus IV-only chemotherapy.21 Patients with stage III ovarian cancer and less than 1 cm of residual disease following cytoreductive surgery were randomized to receive either IV cisplatin and paclitaxel or IV paclitaxel with IP cisplatin and paclitaxel (TABLE 3). Median OS was extended by nearly 16 months in the IP group, a statistically significant improvement. However, IP administration carries the risk of additional complications (i.e., catheter complications, abdominal pain), and only 42% of IP patients completed six cycles of chemotherapy, compared with 83% of IV-only patients. Additionally, during treatment, quality of life was significantly lower in the IP arm. Although there are clear clinical benefits to IP administration, patient selection is important to ensure that this treatment method will be tolerated.
While most patients respond to initial surgery and platinum-based chemotherapy, most will eventually develop recurrent disease. Disease that recurs 6 months or more after initial platinum-based chemotherapy is platinum sensitive, whereas disease that either remains stable during initial treatment or recurs less than 6 months after completion is platinum resistant. Patients with platinum-sensitive disease should receive platinum-based combination therapy as second-line treatment.22 Platinum-resistant disease indicates a poor prognosis and is challenging to treat. These patients may be enrolled in a clinical trial or receive nonplatinum single agents such as liposomal doxorubicin, topotecan, paclitaxel, or others.23 Data are insufficient to recommend one agent over another, and most patients receive multiple lines of single-agent therapy. Systemic chemotherapy is not curative, and the goal of recurrence therapy is to reduce symptoms and improve quality of life.23
Targeted Therapy
Substantial research is being devoted to finding more effective treatments for patients diagnosed with ovarian cancer. In an effort to improve survival and overcome chemoresistance to standard treatments, much research has focused on therapies that target specific cellular pathways. Bevacizumab, which targets the angiogenesis pathway, has demonstrated efficacy both as a single agent and in combination with chemotherapy for recurrent ovarian cancer.24,25 The place of bevacizumab in upfront treatment is less clear. A recently published phase III clinical trial of carboplatin plus paclitaxel with or without bevacizumab in previously newly diagnosed patients demonstrated improved PFS, but no prolongation of OS or improvement in quality of life.26
Another target of interest is poly(ADP-ribose) polymerase (PARP), an enzyme involved in the repair of DNA single-strand breaks. Inhibition of the PARP enzyme impairs this mechanism and may lead to double-strand DNA breaks in tumor cells, particularly in patients with BRCA mutations.27 A recent phase II clinical trial evaluating olaparib, a PARP enzyme inhibitor, in patients with relapsed ovarian cancer demonstrated a significant improvement in PFS compared with placebo (8.4 months vs. 4.8 months, respectively).28 Unfortunately, improvement in OS has not been shown, although data are immature and the final analysis is not yet available.
Ongoing clinical trials are assessing many other cellular targets, such as platelet-derived growth factor, folate receptor alpha, mammalian target of rapamycin, and the phosphatidylinositide 3´-kinase/Akt pathway.27 It is hoped that these novel targeted therapies will positively impact the management of ovarian cancer by providing more individualized options for patients.
CONCLUSION
While much research has been devoted to the treatment of ovarian cancer, mortality has changed little over the last 40 years. Cytoreductive surgery followed by platinum-based combination chemotherapy is the current standard of care, and IP administration of chemotherapy should be strongly considered in eligible patients. Efforts should be made to improve screening methods and diagnose patients at earlier stages. Many clinical trials are ongoing to identify novel effective therapies that can improve outcomes in patients with ovarian cancer.
REFERENCES
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer
Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute.
Based on November 2012 SEER data submission, posted April 2013 to:
http://seer.cancer.gov/csr/1975_2010. Accessed May 29, 2013.
3. Chan JK, Cheung MK, Husain A, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108(3 pt 1):521-528.
4. Epithelial ovarian cancer. In: Hoffman B, Schorge J, Schaffer J, et al, eds. Williams Gynecology. 2nd ed. McGraw-Hill Professional; 2012:853-878.
5. Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3-10.
6. Morch LS, Lokkegaard E, Andreasen AH, et al. Hormone therapy and ovarian cancer. JAMA. 2009;302:298-305.
7. Lin HW, Tu YY, Lin SY, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12:900-904.
8. Cooper A, DePriest P. Surgical management of women with ovarian cancer. Semin Oncol. 2007;34:226-233.
9. Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99:447-461.
10. Chi DS, Bristow RE, Armstrong DK, Karlan BY. Is the easier way ever the better way? J Clin Oncol. 2011;29:4073-4075.
11. Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky
advanced stage ovarian, tubal, and peritoneal carcinoma treated with
primary debulking surgery (PDS) during an identical time period as the
randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol. 2012;124:10-14.
12. Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953.
13. Colombo PE, Mourregot A, Fabbro M, et al. Aggressive surgical
strategies in advanced ovarian cancer: a monocentric study of 203 stage
IIIC and IV patients. Eur J Surg Oncol. 2009;35:135-143.
14. Dewdney SB, Rimel BJ, Reinhart AJ, et al. The role of neoadjuvant
chemotherapy in the management of patients with advanced stage ovarian
cancer: survey results from members of the Society of Gynecologic
Oncologists. Gynecol Oncol. 2010;119:18-21.
15. Rauh-Hain JA, Rodriguez N, Growdon WB, et al. Primary debulking
surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann Surg Oncol. 2012;19:959-965.
16. Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in
stage I and stage II epithelial ovarian cancer. Results of two
prospective randomized trials. N Engl J Med. 1990;322:1021-1027.
17. Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel in
patients with optimally resected stage III ovarian cancer: A Gynecologic
Oncology Group study. J Clin Oncol. 2003;21:3194-3200.
18. Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial
of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line
chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682-1691.
19. Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel
once a week in combination with carboplatin every 3 weeks for advanced
ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331-1338.
20. Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian
cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006;24:988-994.
21. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
22. Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus
platinum-based chemotherapy versus conventional platinum-based
chemotherapy in women with relapsed ovarian cancer: The
ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
23. Fung-Kee-Fung M, Oliver T, Elit L, et al. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol. 2007;14:195-208.
24. Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab
in persistent or recurrent epithelial ovarian cancer or primary
peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165-5171.
25. Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized,
double-blind, placebo-controlled phase III trial of chemotherapy with or
without bevacizumab in patients with platinum-sensitive recurrent
epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039-2045.
26. Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483.
27. Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167-181.
28. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382-1392.
29. Pignata S, Scambia G, Ferrandina G, et al. Carboplatin plus
paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as
first-line treatment for patients with ovarian cancer: the MITO-2
randomized phase III trial. J Clin Oncol. 2011;29:3628-3635.
To comment on this article, contact rdavidson@uspharmacist.com.





