US Pharm. 2014;39(3)(Specialty&Oncology suppl):7-11.
ABSTRACT: Nausea and vomiting, which are experienced by many cancer patients undergoing chemotherapy (ChT), remain a therapeutic challenge of modern medicine. Prevention of nausea and vomiting is an absolute requirement, not only to ensure patients’ functional ability and proper nutrition, but also to help patients adhere to anticancer treatment. Through a better understanding of the biology of emesis, different therapeutic agents have been developed to prevent ChT-induced nausea and vomiting. These agents act on different neuronal pathways that relay afferent signals to the emetic enter of the brain. Because different molecular targets are used, these agents can be used in combination when needed, without adverse effects. Overall, nausea and vomiting caused by ChT can be effectively controlled by antiemetic agents.
Cytotoxic chemotherapeutic agents are notorious for their ability to cause various adverse effects (AEs) in patients with cancer. Nausea and vomiting are the most common and debilitating AEs associated with these drugs. Since DNA-damaging agents remain standard treatment for different types of cancer, supportive care, including the management of chemotherapy (ChT)–induced nausea and vomiting (CINV), poses a constant challenge in the practice of oncology. In this regard, pharmacists, with their up-to-date knowledge, skills, and aptitude, can play a pivotal role in mitigating drug-related AEs.
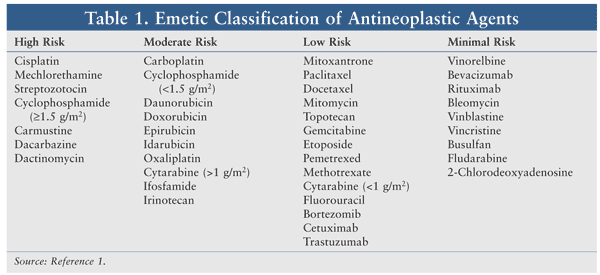
Commonly used chemotherapeutic agents have been classified based on their emetic risk (TABLE 1).1 High-risk agents cause emesis in more than 90% of patients, and moderate-risk and low-risk agents cause emesis in 30% to 90% and 10% to 30% of patients, respectively. In addition to the emetogenic potential of an agent, other factors involved in CINV are medication dosage, route of administration, schedule, female gender, age under 50 years, severe anxiety, and previous history of CINV.1
Two phases are possible with CINV. Acute CINV occurs within the first 24 hours after ChT, whereas delayed CINV begins 24 hours after ChT and can last for up to 5 days.
Central and peripheral neuronal pathways are involved in CINV.2 The putative emetic center located in the medulla receives afferent signals from the chemoreceptor trigger zone (CTZ), cerebral cortex, vestibular apparatus, and visceral organs (e.g., the intestine) and vasculature.2 Neurotransmitters such as dopamine, serotonin, substance P, and endocannabinoids are important players in this process. Of these neurotransmitters, only cannabinoids produce an antiemetic effect; the others have proemetic functions. The agents currently used to treat CINV were developed to target receptors of the neurotransmitters involved in emesis, with the goal of preventing transmission of afferent signals to the emetic center.
FIRST-LINE ANTIEMETICS
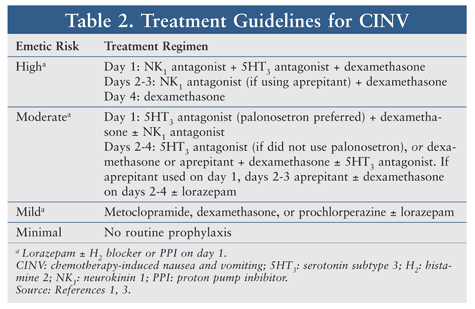
According to current treatment guidelines for the prevention of CINV, first-line antiemetic drugs include 5-HT3 (serotonin) receptor antagonists, neurokinin-1 (NK1) receptor antagonists, and dexamethasone (TABLE 2).3
5-HT3 Receptor Antagonists
These agents block the receptor-mediated actions of the serotonin released from the enterochromaffin cells of the gastrointestinal mucosa, thereby preventing the initiation of neuronal signals to the central nervous system (CNS) through vagal and spinal afferents. 5-HT3 receptor antagonists also block 5-HT3 receptors in the CTZ and other central structures, such as the nucleus tractus solitarius.3 Four 5-HT3 receptor antagonists are currently being used in the United States: ondansetron, granisetron, dolasetron, and palonosetron. Palonosetron, the newest agent, binds to 5-HT3 receptors with greater affinity. Dolasetron is converted in the plasma to its active metabolite, hydrodolasetron.
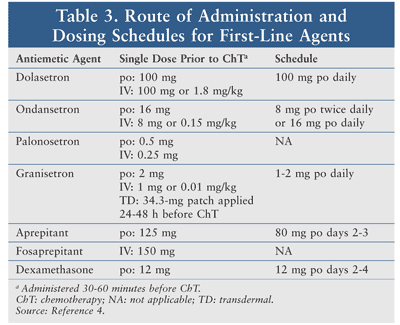
These agents are well tolerated, with the most common AEs being headache, fatigue, and constipation. There is an increased risk of QTc prolongation with dolasetron, ondansetron, and granisetron. No significant drug-drug interactions have been reported with these agents. See TABLE 3 for route of administration and dosing schedules.4
NK1 Receptor Antagonist
Aprepitant blocks the action of substance P, a tachykinin peptide in the brain and periphery. Activation of the NK1 receptor (a Gq protein–coupled receptor) results in inositol triphosphate synthesis and calcium mobilization. Fosaprepitant, a phosphate prodrug of aprepitant, is converted to the parent drug following IV administration. AEs of aprepitant include fatigue, alopecia, dizziness, hiccups, and diarrhea. Aprepitant, a strong dose-dependent inhibitor of CYP3A4, should be used with caution with medications that are metabolized primarily by the enzyme. Aprepitant is a substrate and inducer of CYP3A4; it also induces CYP2C9 and consequently decreases the efficacy of warfarin and hormonal contraceptives.3
Dexamethasone
Corticosteroids have been widely used for CINV prevention for decades. Dexamethasone is the most frequently used agent in the class. Dexamethasone’s site and mechanism of action in CINV are not fully understood. As an anti-inflammatory agent, dexamethasone may suppress prostaglandin activity in the CNS. At the cellular level, the drug must enter its target cell to bind to its cognate receptor in the cytoplasm; the drug-receptor complex then enters the nucleus, undergoes receptor dimerization, and binds to glucocorticoid-responsive elements to modulate transcription. During short-term treatment, glucocorticoids can cause insomnia, dyspepsia, hyperglycemia, agitation, increased appetite, and weight gain. A long-term AE of glucocorticoids is immunosuppression.3
IMPORTANT CLINICAL TRIALS OF FIRST-LINE ANTIEMETICS
Trials of 5-HT3 Receptor Antagonists
Comparison of Different Agents in Therapeutic Class: In a multicenter, double-blind, parallel-group trial, a single IV dose of ondansetron (8 mg or 32 mg) was compared with a single IV dose of granisetron (3 mg) for the control of cisplatin-induced acute emesis.5 Of 496 randomized patients, 165 received ondansetron 8 mg, 162 received ondansetron 32 mg, and 169 received granisetron 3 mg prior to cisplatin administration. Zero emetic episodes were reported in 59% and 51% of patients in the ondansetron 8-mg and 32-mg groups, respectively. Emesis was completely controlled in 56% of granisetron patients. Seventy-six percent and 74% of patients receiving low-dose (LD) and high-dose (HD) ondansetron, respectively, experienced two or fewer emetic episodes; 78% of granisetron patients attained this outcome. A nausea level of “none” or “mild” was achieved by 71% and 69% of LD and HD ondansetron patients, respectively, and by 73% of granisetron patients. Headache was the most common AE, and no severe AEs were reported.
In a prospective, randomized, open trial, the antiemetic effects of ondansetron and granisetron were compared in patients receiving highly emetogenic ChT (Study 1) and in patients receiving moderately emetogenic ChT (Study 2).6 In Study 1, patients (n = 182) on regimens containing cisplatin (>70 mg/m2) received either IV ondansetron (24 mg) or IV granisetron (3 mg). In Study 2, patients (n = 164) received either ondansetron 16 mg IV or granisetron 3 mg IV to prevent acute emesis. In Study 1, complete response (CR) (no vomiting; nausea possible) was comparable between ondansetron and granisetron patients (52% vs. 49%). No marked difference was noted between ondansetron and granisetron patients in Study 2.
A phase III comparative trial examined the safety and efficacy of single-dose palonosetron versus single-dose ondansetron in preventing CINV following highly emetogenic ChT.7 Patients were administered a single fixed IV dose of either palonosetron (0.75 mg or 0.25 mg) or ondansetron (32 mg) 30 minutes before ChT on day 1. Dexamethasone prophylaxis was allowed at the investigators’ discretion. Palonosetron 0.25 mg and 0.75 mg were at least as effective as ondansetron in preventing acute CINV (respective CR rates were 59.2%, 65.5%, and 57%). Parenteral dexamethasone was administered to 447 patients. For both delayed effect and overall effect, patients pretreated with dexamethasone plus palonosetron 0.25 mg had significantly higher CR rates than those pretreated with dexamethasone and ondansetron (42% vs. 28.6% and 40.7% vs. 25%, respectively).
In a randomized, double-blind trial, the therapeutic efficacy and AEs of oral single-dose dolasetron versus a multiple-dose regimen of oral ondansetron were compared in 399 cancer patients receiving moderately emetogenic ChT.8 A single oral dose of dolasetron 25, 50, 100, or 200 mg was administered 1 hour prior to ChT. The first dose of oral ondansetron (8 mg) was administered 1.5 hours prior to ChT, and another three doses were administered every 8 hours after ChT. Dolasetron produced a dose-dependent effect in attaining CR (no emetic episodes and no use of escape antiemetics). The single dose of dolasetron 200 mg was found to be therapeutically equivalent to the multiple-dose ondansetron regimen. Other than headache, no major AEs were reported.
In a phase III, multicenter, randomized, double-blind, stratified, parallel-group, active-comparator study, palonosetron was compared with granisetron for CINV in patients receiving highly emetogenic ChT (cisplatin or an anthracycline antibiotic plus cyclophosphamide combination).9 Both antiemetics were administered with dexamethasone. Single-dose palonosetron (0.75 mg) or granisetron (40 mcg/kg) was administered to patients 30 minutes prior to ChT on day 1. On day 1, both treatment groups also received IV dexamethasone 16 mg. On days 2 and 3, cisplatin patients received IV dexamethasone 4 mg, and anthracycline-cyclophosphamide patients received oral dexamethasone 4 mg. Study endpoints were CRs during the acute and delayed phases of ChT. In the acute phase, 75.2% of cisplatin patients had CR, compared with 73.3% of granisetron patients. A total of 315 palonosetron patients (56.8%) had CR in the delayed phase, which was significantly higher than the rate in granisetron patients (44.5%). Major AEs were constipation and elevated aminotransferases.
Comparison of Two Different Granisetron Formulations: In this study, the efficacy and tolerability of granisetron transdermal delivery system (GTDS), a novel transdermal formulation for continuous 7-day drug delivery, were compared with those of oral granisetron for prevention of CINV.10 Patients received either one GTDS patch or oral granisetron (2 mg/day for 3-5 days) 7 days prior to multiple-day ChT. The primary outcome was complete control of CINV from ChT initiation to 24 hours following the last ChT administration. Complete control was achieved in 60% of GTDS patients and 65% of oral granisetron patients.
Aprepitant Trials
In a multicenter, randomized, placebo-controlled, parallel-group, phase III trial, patients were treated with one of two antiemetic regimens prior to undergoing high-dose cisplatin therapy.11 Patients assigned to the standard antiemetic regimen received IV ondansetron and oral dexamethasone on day 1, followed by dexamethasone on days 2 and 4. Patients on the investigational regimen received aprepitant, dexamethasone, and ondansetron on day 1, followed by aprepitant and dexamethasone on days 2 and 3; on the last day (day 4), they received dexamethasone only. Aprepitant patients had markedly better control of emesis compared with those who received the standard regimen, and AEs were similar in both groups.
In a different trial, ChT-naïve patients scheduled to receive cisplatin-based ChT were randomized to standard therapy or an aprepitant regimen.12 Standard therapy consisted of dexamethasone and ondansetron on day 1, then dexamethasone on days 2 and 4. The aprepitant regimen consisted of aprepitant plus dexamethasone plus ondansetron on day 1, followed by dexamethasone and aprepitant on days 2 and 3, and dexamethasone only on day 4. The primary outcome was CR (no emesis and no rescue medication) from day 1 through day 5. CR was significantly higher in patients receiving the aprepitant regimen than in those on the standard regimen (72.7% vs. 52.3%), suggesting better control of CINV in both acute and delayed phases.
Dexamethasone Trials
In one trial, ChT-naïve patients received IV granisetron, with or without IV dexamethasone, prior to cisplatin treatment.13 Oral granisetron was administered at 6 hours and 12 hours post ChT. At the end of 24 hours, patients who received dexamethasone had significantly higher complete protection from emesis, compared with patients who had not taken the steroid (64% vs. 34%).
A double-blind, randomized crossover study compared the antiemetic effects of ondansetron and ondansetron plus dexamethasone in 102 chemotherapy-naïve patients scheduled to receive cisplatin at a dosage ≥50 mg/m2.14 Protection from nausea and emesis was significantly higher in the ondansetron-plus-dexamethasone group, compared with the ondansetron-alone group. Furthermore, among patients who indicated treatment preferences, 74% preferred ondansetron plus dexamethasone, versus 26% for ondansetron only (P <.003).
SECOND-LINE AGENTS
Benzodiazepines
These agents are used adjunctively against CINV.3 Benzodiazepines, centrally acting CNS depressants used to treat anxiety, insomnia, muscle spasm, seizure disorders, and alcohol disorders, do not produce a direct inhibitory effect on nausea and vomiting; however, their anxiolytic, sedative, and amnestic properties are useful when these drugs are administered together with antiemetics. Benzodiazepines increase the inhibitory effects of gamma-aminobutyric acid (GABA) by increasing the frequency of the opening of chloride channels in the GABA-chloride ionophore present in the brain.3 Lorazepam, the agent most widely used to prevent CINV, may be given orally, intramuscularly, intravenously, or sublingually. When given to patients receiving cytotoxic ChT and prochlorperazine, lorazepam decreases the severity and duration of nausea, the severity of vomiting, and the number of vomiting episodes.15 AEs associated with benzodiazepine use include CNS depression, anterograde amnesia, respiratory depression, and paradoxical psychological reactions.3 Lorazepam is metabolized into inactive compounds in the liver and is excreted by the kidneys.
Metoclopramide
Before the introduction of serotonin antagonists, metoclopramide was widely used for CINV. It is no longer a first-line agent for this purpose. Chemically a substituted benzamide, metoclopramide given at therapeutic doses blocks dopaminergic D2 receptors.3 At higher doses, it also inhibits serotonergic 5-HT3 receptors. Metoclopramide is believed to act both centrally and peripherally. The drug also produces a prokinetic effect by enhancing the rate of gastric emptying, an effect that may contribute to its antiemetic effects. Metoclopramide is associated with serious CNS AEs, including dystonia, akathisia, and extrapyramidal side effects (EPS). Diphenhydramine and lorazepam may be needed to minimize the severity of these AEs.
Dronabinol and Nabilone
Dronabinol and nabilone are cannabinoids that stimulate cannabinoid type 1 receptors in and around the emetic center.3 These agents are effective against moderately emetogenic ChT, but insufficient data exist regarding benefits of their use.16 AEs of cannabinoids include euphoria, dysphoria, dizziness, paranoid reactions, and orthostatic hypotension. The occurrence of these AEs has discouraged the use of cannabinoids in treating CINV.
Prochlorperazine
A piperazine derivative, prochlorperazine is a centrally acting dopaminergic antagonist that is classified as a first-generation antipsychotic agent for treating schizophrenia. Because this drug blocks dopaminergic signaling in the CTZ, it also may be used to prevent nausea and vomiting in patients scheduled to receive ChT.3
Olanzapine
Olanzapine, an antagonist of serotonin and dopamine receptors, is a second-generation antipsychotic widely used to treat schizophrenia. Unlike the first-generation antipsychotics, olanzapine does not produce EPS, but it has been associated with an increased risk of metabolic disturbances (i.e., hyperlipidemia, hyperglycemia, weight gain).3 In a randomized, placebo-controlled trial, olanzapine and aprepitant were compared for CINV prevention in patients receiving highly emetogenic ChT.17 A total of 241 patients were included in the final evaluation. All patients were treated with palonosetron on day 1. Patients in the olanzapine group (n = 120) received olanzapine 10 mg daily for 4 days (days 1-4), and dexamethasone on day 1 only. In the aprepitant group (n = 121), patients received aprepitant on days 1 through 3 (125 mg on day 1, then 80 mg/day thereafter) and dexamethasone on days 1 through 4. In the olanzapine group, CRs (no emesis, no nausea) reported for the acute period, delayed period, and overall period were 97%, 77%, and 77%, respectively. Although these effects were not statistically significant compared with those for the aprepitant group, the olanzapine regimen was comparable in antiemetic efficacy. Also, nausea control was better achieved by the olanzapine-containing regimen.
Gabapentin
Interestingly, the anticonvulsant gabapentin, when added to an antiemetic regimen consisting of ondansetron, dexamethasone, and ranitidine, produced a significant increase in the rate of CR (65%, vs. 42.5% in the nongabapentin group).18 In this pilot study, gabapentin was administered at a dosage of 300 mg orally. AEs were found not to be markedly different from those experienced by the group not receiving gabapentin.
CONCLUSION
First-line antiemetic agents (serotonin antagonists, NK1 antagonists, and corticosteroids) have been proven to be quite effective in treating patients for CINV. In addition, AEs produced by these drugs are usually well-tolerated. These agents, therefore, should be carefully selected and individualized to provide optimum relief to the patient in a cost-effective manner. Meanwhile, promising antiemetic agents, such as olanzapine and pregabalin, should undergo further evaluation.
REFERENCES
1. Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of
Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932-2947.
2. Cubeddu LX. Mechanisms by which cancer chemotherapeutic drugs induce emesis. Semin Oncol. 1992;19(6 suppl 15):2-13.
3. Thompson LA, O’Bryant CL. Chemotherapy-induced nausea and vomiting: guideline summary and clinical challenges. Pharm Pract News. 2013;3:19-25.
4. Scarpace SL. Supportive care in oncology. In: Chisholm-Burns MA, Wells BG, Schwinghammer TL. Pharmacotherapy Principles and Practice. 3rd ed. New York, NY: McGraw-Hill Professional; 2013:1721-1753.
5. Ruff P, Paska W, Goedhals L, et al. Ondansetron compared with
granisetron in the prophylaxis of cisplatin-induced acute emesis: a
multicentre double-blind, randomised, parallel-group study. The
Ondansetron and Granisetron Emesis Study Group. Oncology. 1994;51:113-118.
6. Gebbia V, Cannata G, Testa A, et al. Ondansetron versus
granisetron in the prevention of chemotherapy-induced nausea and
vomiting. Results of a prospective randomized trial. Cancer. 1994;74:1945-1952.
7. Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III,
double-blind, randomized trial of palonosetron compared with ondansetron
in preventing chemotherapy-induced nausea and vomiting following highly
emetogenic chemotherapy. Ann Oncol. 2006;17:1441-1449.
8. Fauser AA, Duclos B, Chemaissani A, et al. Therapeutic equivalence
of single oral doses of dolasetron mesilate and multiple doses of
ondansetron for the prevention of emesis after moderately emetogenic
chemotherapy. European Dolasetron Comparative Study Group. Eur J Cancer. 1996;32A:1523-1529.
9. Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone
versus granisetron plus dexamethasone for prevention of nausea and
vomiting during chemotherapy: a double-blind, double-dummy, randomised,
comparative phase III trial. Lancet Oncol. 2009;10:115-124.
10. Boccia RV, Gordan LN, Clark G, et al. Efficacy and tolerability
of transdermal granisetron for the control of chemotherapy-induced
nausea and vomiting associated with moderately and highly emetogenic
multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer. 2011;19:1609-1617.
11. Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition
of the neurokinin 1 receptor antagonist aprepitant to standard
antiemetic therapy improves control of chemotherapy-induced nausea and
vomiting. Results from a randomized, double-blind, placebo-controlled
trial in Latin America. Cancer. 2003;97:3090-3098.
12. Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1
antagonist aprepitant for the prevention of chemotherapy-induced nausea
and vomiting: a multinational, randomized, double-blind,
placebo-controlled trial in patients receiving high-dose cisplatin—the
Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112-4119.
13. Latreille J, Stewart D, Laberge F, et al. Dexamethasone improves
the efficacy of granisetron in the first 24 h following high-dose
cisplatin chemotherapy. Support Care Cancer. 1995;3:307-312.
14. Roila F, Tonato M, Cognetti F, et al. Prevention of
cisplatin-induced emesis: a double-blind multicenter randomized
crossover study comparing ondansetron and ondansetron plus
dexamethasone. J Clin Oncol. 1991;9:675-678.
15. Bishop JF, Olver IN, Wolf MM, et al. Lorazepam: a randomized,
double-blind, crossover study of a new antiemetic in patients receiving
cytotoxic chemotherapy and prochlorperazine. J Clin Oncol. 1984;2:691-695.
16. Ahmedzai S, Carlyle DL, Calder IT, Moran F. Anti-emetic efficacy
and toxicity of nabilone, a synthetic cannabinoid, in lung cancer
chemotherapy. Br J Cancer. 1983;48:657-663.
17. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the
prevention of chemotherapy-induced nausea and vomiting: a randomized
phase III trial. J Support Oncol. 2011;9:188-195.
18. Cruz FM, de Iracema Gomes Cubero D, Taranto P, et al. Gabapentin
for the prevention of chemotherapy-induced nausea and vomiting: a pilot
study. Support Care Cancer. 2012;20:601-606.
To comment on this article, contact rdavidson@uspharmacist.com.





