U.S. Pharm. 2008;33(4)(Oncology suppl):20-30.
ABSTRACT: Approximately 75% of postmenopausal women with breast cancer have hormone receptor-positive (HR+) disease.1 Tamoxifen was the standard of therapy for HR+ breast cancer in postmenopausal women until the advent of the selective third-generation aromatase inhibitors, which are anastrozole, letrozole, and exemestane.3,4 These agents have demonstrated superior disease-free survival in clinical trials compared with tamoxifen, but their benefit when used for more than five years in postmenopausal women with HR+ breast cancer has not been established.3,4
It has been estimated that three- fourths of postmenopausal women with breast cancer have hormone receptor-positive (HR+) disease.1 Tamoxifen--a selective estrogen-receptor modulator with estrogenic effects on bone, endometrial tissue, and lipids and anti!= estrogenic effects on breast tissue--was the standard therapy for HR+ breast cancer in postmenopausal women until the introduction of the selective third-generation aromatase inhibitors (AIs).2-4 These agents--anastrozole, letrozole, and exemestane--have demonstrated superior disease-free survival (DFS) compared with tamoxifen in clinical trials.3,4 Adverse effects associated with the use of tamoxifen--endometrial cancer, thromboembolic complications, and tamoxifen resistance--have encouraged the development of therapies with a different mechanism of action, such as the AIs.2
See TABLE 1 for a summary of important information about the third-generation AIs.
Background
The Scottish surgeon George Beatson described the hormonal contribution of estrogens to carcinogenesis in relation to breast cancer in 1896.5 Beatson recognized that removal of the ovaries (oophorectomy) was beneficial in women with inoperable breast cancer.5 This surgically induced state of estrogen deprivation contributed to the inhibition of tumor growth in breast cancer cells in women with HR+ disease.5
Aminoglutethimide, originally formulated as an anticonvulsant agent, was the first nonspecific AI initially promoted in the late 1970s as a second-line agent following tamoxifen for postmenopausal women with advanced HR+ breast cancer.6 Toxic side effects such as adrenal suppression and the necessity for steroid therapy led to the withdrawal of aminoglutethimide from the market.6 In postmenopausal women with early- and late-stage HR+ breast cancer, the third-generation AIs demonstrate a better toxicity profile, more selectivity, higher potency, and improved clinical efficacy compared with the older agents. 6
Classification4,7
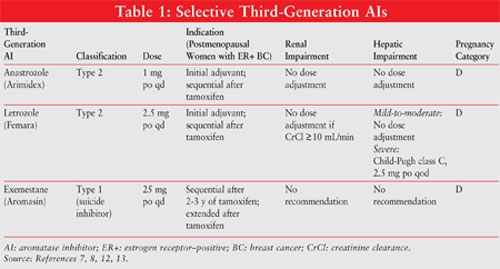
AIs are classified as type 1 steroidal (noncompetitive, irreversible) or type 2 nonsteroidal (competitive, reversible) inhibitors. Exemestane, a type 1 inhibitor, binds irreversibly to the site on the aromatase molecule and is known as a suicide inhibitor. The type 2 inhibitors anastrozole and letrozole bind reversibly to aromatase. See FIGURE 1 for the chemical structures of the different AIs.
Mechanism of Action
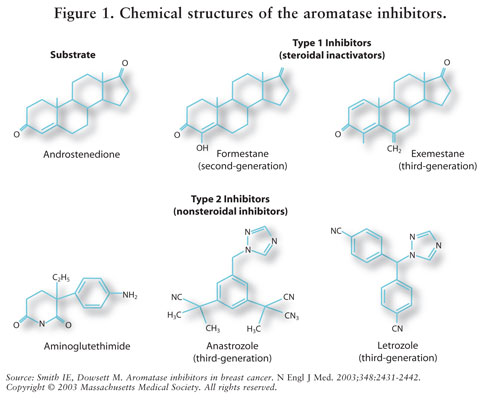
Estrogen synthesis is mediated by the enzyme aromatase.7 Aromatase converts androstenedione and testosterone to estrone and estradiol.5,7 AIs reduce estrogen in postmenopausal women, thereby inhibiting its carcinogenic effect on breast tissue.7 Because the ovaries of premenopausal women are still functioning, AIs are not indicated for this population outside of clinical trials; in these women, AIs would stimulate the hypothalamus and the pituitary to increase production of gonadotropins.4 See FIGURE 2.
Toxicities6,8
Adverse effects of AIs include cardiovascular dysfunction such as hypertension, vasodilation, edema, and chest pain. Further study is required to evaluate the full effect of AIs on cardiac function. Patients may experience decreased bone mineral density (BMD), increased risk of fracture, vasomotor symptoms, vaginal dryness, musculoskeletal pain, headache, insomnia, depression, increased cough, dyspnea, gastrointestinal discomfort, weight gain, and possibly elevated lipid levels. Bone loss is a predominant side effect; bisphosphonates such as zoledronic acid have been used to counteract it. Patients on AIs should receive vitamin D and calcium supplementation. Protracted adverse effects and effects on cognitive function from AI therapy have not yet been determined.9 AIs are contraindicated in pregnant women.
Drug Interactions
Anastrozole inhibits CYP1A2, CYP2C9, and CYP3A4.10,11 Letrozole is metabolized by CYP2A6 and CYP2C19, and exemestane metabolizes CYP3A4.8,10-13 Inducers of CYP3A4 such as rifampin, phenytoin, carbamazepine, and St. John's wort may cause a decrease in exemestane levels when given concomitantly. 8,12,13 Tamoxifen causes a 38% decrease in letrozole and a 27% decrease in anastrozole when co-administered.2,7,8,14
Indications and Dosing
Anastrozole 10,11: Anastrozole 1 mg once daily is indicated for early breast cancer as initial adjuvant therapy in postmenopausal women with hormonally sensitive disease and as first line for HR+ or HR-unknown metastatic or locally advanced disease. It also is approved for use following tamoxifen therapy in women with progressive disease. Dose adjustment is not required for renal impairment or mild-to-moderate hepatic impairment.
Letrozole11,12: Letrozole 2.5 mg once daily is indicated for postmenopausal women with HR+ early-stage breast cancer as initial therapy or as extended adjuvant therapy following tamoxifen. It also is indicated for HR+ or HR-unknown metastatic disease. Dose adjustments are recommended for severe hepatic impairment.
Exemestane11,14: Exemestane 25 mg po once daily is indicated as adjuvant therapy two to three years following tamoxifen for postmenopausal women with HR+ early breast cancer, for a total of five consecutive years of hormonal therapy. It also may be used for disease that has progressed after tamoxifen therapy. It should be taken after a meal. Dose adjustment is unnecessary for hepatic or renal impairment
Clinical Trials
Tamoxifen-related toxicities such as endometrial cancer prompted the initiation of large-scale clinical trials investigating the use of third-generation AIs in postmenopausal women with HR+ breast cancer. Various trial designs were used to assess the performance of these agents compared with tamoxifen.6 Superior outcomes in terms of clinical effectiveness and toxicity profiles established AIs as a new standard of adjuvant therapy in postmenopausal women with HR+ breast cancer.6
ATAC15: ATAC (Arimidex, Tamoxifen Alone or in Combination), a multicenter, randomized, double-blind study, compared three treatment arms: tamoxifen 20 mg + anastrozole placebo, anastrozole 1 mg + tamoxifen placebo, and a combination of tamoxifen 20 mg + anastrozole 1 mg. The primary objective was to evaluate the safety and efficacy of tamoxifen versus anastrozole and the combination of anastrozole and tamoxifen over five years. A total of 9,366 patients--3,125 in the anastrozole arm, 3,116 in the tamoxifen arm, and 3,125 in the combination arm--were treated for a median of 33 months.
After three years, anastrozole demonstrated DFS of 89.4% versus 87.4% for tamoxifen (hazard ratio [HR] 0.83, 95% CI [0.71-0.96], P =.013). Anastrozole decreased the rate of contralateral breast cancer (CLBC) compared with tamoxifen (odds ratio [OR] 0.42, 95% CI [0.22-0.79], P =.007). The combination arm was closed because no clinical benefit beyond tamoxifen was found. Anastrozole was associated with adverse effects such as endometrial cancer (P =.02), vaginal bleeding, discharge (P <.0001), cerebrovascular events (P =.0006), venous thromboembolic events (P =.0006), and hot flashes (P <.0001); tamoxifen was associated with fewer fractures and less joint pain (P <.0001). After 68 months, anastrozole increased DFS (HR 0.87, 95% CI [0.78-0.97], P =.01) and time to recurrence (HR 0.79, 95% CI [0.70-0.90], P =.0005) and decreased distant metastases (HR 0.86, 95% CI [0.74-0.99], P =.04) and CLBC (42% decrease, P =.01).16 After a median follow-up interval of 100 months, HR+ patients on anastrozole demonstrated improvements in DFS (HR 0.85; P =.003), occurrence of CLBC (HR 0.60, P =.004), time to recurrence (HR 0.76, P =.0001), and time to distant recurrence (HR 0.84, P =.022) compared with patients on tamoxifen. The absolute difference in recurrence rates improved from 2.8% after five years to 4.8% after nine years. Anastrozole fracture rates were higher during therapy, but the difference decreased substantially after the 100-month follow-up period. The extended-therapy period had a risk of fracture of 1.15 with anastrozole versus 1.02 with tamoxifen. The risk of endometrial cancer was reduced on and off anastrozole therapy compared with tamoxifen, with an annual rate of 0.043 versus 0.14 and 0.014 versus 0.12, respectively. A difference in overall survival (OS) has not been identified.
MA.1712,17: MA.17 was a randomized, placebo-controlled, double-blind trial assessing the effectiveness of five years of letrozole therapy in postmenopausal women with breast cancer who had already completed five years of adjuvant tamoxifen therapy. Patients received letrozole 2.5 mg or placebo daily for five years. The primary endpoint was DFS; secondary endpoints were quality of life, extended safety profile, and OS. Of the 5,187 patients, 2,593 received letrozole and 2,594 received placebo; median follow-up was 2.4 years.
In the preliminary analysis there were 207 local or metastatic recurrences of breast cancer or initial malignancies of CLBC, 75 in the letrozole group and 132 in the placebo group. Projected four-year DFS ratesÜ for the two groups were 93% and 87%, respectively (P ?.001 for evaluation of DFS). In the letrozole arm, DFS had an HR of 0.61 (95% CI [0.47-0.79], P ?.001). Letrozole demonstrated a 43% decrease in breast cancer incidence (HR 0.57, 95% CI [0.43-0.75], P =.00008).4 With the exception of node-positive patients (P =.04), OS did not reach statistical significance (HR 0.82, P =.30).4 Patients in the letrozole arm experienced low-grade vasomotor symptoms, myalgia, and arthritis. Osteoporosis was more common (although not significantly) in the letrozole arm versus placebo (5.8% vs. 4.5%; P =.07), and fracture rates were comparable between arms. The study was closed after a median of 2.4 years because of a decrease in breast cancer events in the letrozole arm. Extended adjuvant therapy with letrozole after completion of five years of tamoxifen improved DFS.
BIG 1-987,9,18: The Breast International Group (BIG) 1-98 study, a double-blind, multicenter, randomized trial, enrolled 8,028 postmenopausal women with HR+ early-stage breast cancer. The study comprised four treatment arms that evaluated five years of tamoxifen monotherapy, five years of letrozole monotherapy, tamoxifen for two years then a switch to letrozole for three years, or letrozole for two years then a switch to tamoxifen for three years. The primary objective was DFS; secondary objectives were time to distant metastasis, safety, and OS. DFS increased in patients who received letrozole compared with those who received tamoxifen (HR 0.81, 95% CI [0.70-0.93], relative reduction 19%, P =.003), with an absolute difference of 1.5% at three years. Tamoxifen increased the occurrence of thromboembolic events compared with letrozole (OR 0.38, P <.0001). Bone fractures were increased in the letrozole arm (OR 1.44, P =.006).
IES14,19: The IES (Intergroup Exemestane Study) was a randomized, double-blind, multicenter, Phase III study whose objective was to determine whether changing to exemestane after two to three years of tamoxifen was more effective than continuing tamoxifen for five years. The trial enrolled 4,742 postmenopausal women randomized to two treatment arms; 2,362 subjects were scheduled to switch to exemestane 25 mg po qd after two to three years of tamoxifen 20 mg po qd to complete five years of therapy, and the remaining 2,380 were scheduled to receive tamoxifen 20 mg po qd for five years. DFS was the primary endpoint; secondary endpoints were OS, extended toxicity profile, and frequency of CLBC.
Adverse events, which occurred over a median of 30.6 months, included frequency of CLBC, local or metastatic recurrence, and death. There were 449 events--183 in the exemestane arm and 266 in the tamoxifen arm. The exemestane arm had an unadjusted HR of 0.68 (95% CI [0.56-0.82], P <.001 by the log-rank test), which demonstrated a 32% decrease in risk and was analogous to an advantage in DFS of 4.75 (95% CI [2.6-6.8]) at three years. After three years, the exemestane arm had a DFS of 91.5% (95% CI [90.0 to 92.7]) compared with 86.8% (95% CI [85.1-88.3]) in the tamoxifen arm. There was no significant difference in OS between the two groups, with 93 deaths in the exemestane arm and 106 deaths in the tamoxifen arm. There was a significant difference in the frequency of CLBC (P =.04)--20 subjects in the tamoxifen arm and nine in the exemestane arm. The use of exemestane to complete the five years of treatment after two to three years of tamoxifen improved DFS compared with five years of tamoxifen.
Preventing Bone Loss from AI Therapy20
Postmenopausal women are prone to developing decreased BMD because of reduced levels of estrogen. AI therapy in the postmenopausal breast cancer patient exacerbates this tendency. Denosumab, a fully human monoclonal investigational antibody, binds receptor activator for nuclear factor kB ligand (RANKL) and prevents RANKL activity. RANKL is vital for osteoclastic activity, which means the breakdown of bone. Denosumab and risedronate, a bisphosphonate, are being evaluated for their ability to counteract the bone loss associated with AI therapy.
Denosumab Study20: A multicenter, randomized, Phase III study evaluated the use of denosumab 60 mg administered subcutaneously once every six months in postmenopausal women taking AIs for HR+ nonmetastatic breast cancer. The objective was to assess the change in lumbar-spine BMD from baseline to 12 months. Of the 252 patients, 127 were assigned to denosumab and 125 were assigned to placebo. Both arms received calcium and vitamin D. BMD was evaluated by dual-energy x-ray absorptiometry. After 12 and 24 months of therapy with denosumab or placebo, there was a difference of 5.5% and 7.6%, respectively, in BMD increase in the lumbar-spine region in favor of denosumab (P <.0001 for both months). Denosumab demonstrated a comparable toxicity profile relative to placebo. Denosumab therapy for more than 24 months has not yet been assessed.
IBIS-II Bone Sub-Study21: The International Breast Cancer Intervention Study-II (IBIS-II) Bone Sub-Study was derived from IBIS-II, which evaluated postmenopausal women with an increased probability of developing breast cancer who were randomized to anastrozole or placebo for five years. The substudy, with a current enrollment of 700 patients, is evaluating risedronate versus placebo for the prevention of BMD loss in women with low T-scores at baseline. A subset of patients from the IBIS-II trial were assigned to one of three different treatment strategies in the sub-study. After one year of therapy, 350 anastrozole and 350 placebo patients from IBIS-II received substudy results. Stratum 1 comprised 227 patients on anastrozole with normal T-scores (T-score ?1), stratum 2 comprised 80 patients with osteopenia (ñ2.5 ? T-score <1) who were assigned to risedronate 35 mg once weekly or placebo, and stratum 3 comprised 43 patients with osteoporosis (ñ4 <T-score <2.5) who were assigned to risedronate 35 mg once weekly.
For patients with osteopenia or osteoporosis at baseline, risedronate appeared to inhibit bone loss associated with anastrozole. In stratum II, risedronate demonstrated favorable effects on BMD preservation for the total hip but not the lumbar spine (P =.005 and P =.15, respectively, vs. placebo). No serious adverse effects or drug interactions were associated with risedronate.
Trial of AIs in Premenopausal Women (SOFT)21-26
Approximately 33% of women under the age of 50 will be diagnosed with invasive breast cancer. 22 The Suppression of Ovarian Function Trial (SOFT, IBCSG 24-02, BIG 2-02) is investigating the use of AI therapy in premenopausal women with HR+ breast cancer. The trial, which has a targeted patient enrollment of 3,000, involves premenopausal women with HR+ breast cancer who have completed chemotherapy or who have received surgery only. Ovarian ablation is established through administration of triptorelin (a gonadotropin-releasing hormone) once monthly for five years, surgical oophorectomy, or pelvic irradiation. Patients will be randomized to one of three arms, with the first arm receiving tamoxifen once daily for five years, the second arm receiving tamoxifen for five years in combination with ovarian ablation, and the third arm receiving exemestane for five years in combination with ovarian ablation. Patients will receive exemestane or tamoxifen for five years.
Chemoprevention Trials23-27
Tamoxifen is indicated for breast cancer chemoprevention.6 Its toxicity profile is a cause of constraint with regard to therapeutic use.6 The AIs may reduce the incidence of HR+ breast cancer in high-risk patients because of a decrease in the incidence of CLBC compared with tamoxifen in clinical trials such as ATAC.6
AI chemoprevention studies include the International Breast Cancer Study Group (IBCSG) trial 31-03 (IBCSG 31-03) and the National Cancer Institute of Canada's MAP.3 trial. The primary objective of the multicenter, international, randomized, placebo-controlled IBCSG 31-03 trial is to evaluate the use of anastrozole for the prevention of breast cancer in postmenopausal women. Its primary outcome measure is the incidence of invasive and noninvasive breast cancer; the secondary outcome measure is the incidence of osteoporosis and fractures.5 The IBCSG 31-03 trial has a target enrollment of 6,000. The MAP.3 trial evaluates exemestane as a chemopreventive agent in postmenopausal women. The primary objective of the placebo-controlled MAP.3 trial is to assess the effectiveness of exemestane in decreasing the incidence of invasive breast cancer.
Potential Future Uses: Case Reports
Letrozole was used after failure of clomiphene to induce fertility in 22 women with polycystic ovary syndrome. Letrozole induced ovulation in 75% of cycles versus 44.4% of cycles induced by clomiphene. Four pregnancies resulted from the ovulations induced by letrozole, and no significant adverse effects were associated with letrozole.28,29
A 57-year-old obese woman with endometriosis following hysterectomy and bilateral salpingo-oophorectomy received anastrozole after megestrol therapy. The patient had no endometrial lesions after nine months.28,30
A patient with McCune-Albright's syndrome was given anastrozole for premature puberty; the patient's estradiol normalized after 2.5 years of anastrozole and the bone age advanced by six months.28,31 Increased estrogen may induce gynecomastia and hypogonadotropic hypogonadism.28,31 Decreasing estrogen with an AI resulted in normal gonadotropin and testosterone in males with gynecomastia and hypogonadotropic hypogonadism. 28,31,32
Letrozole was studied in patients with relapsed ovarian cancer; those patients who received letrozole had stabilization of disease.28,33 Neoadjuvant letrozole was given to 10 postmenopausal women with endometrial cancer; the drug demonstrated constructive clinical changes and had no negative surgical impact.28,34
When treatment with testolactone versus anastrozole was compared in infertile men, the efficacy of the two agents was similar.28,35 Patients with Klinefelter's syndrome had more effective treatment with testolactone than with anastrozole.28,35
Conclusions
The third-generation AIs have shown superior DFS in postmenopausal women with HR+ breast cancer, but the clinical benefit of more than five years of AI therapy in these patients has not been established.4 The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-33 (exemestane) trial and a subsequent randomization of the MA.17 (letrozole) trial will examine the use of these AIs versus placebo for an additional five years.4 AIs are an appropriate alternative for HR+ postmenopausal women in whom tamoxifen is contraindicated.4
The beneficial effects of the third-generation AIs include a more tolerable toxicity profile and superior DFS compared with tamoxifen. The level of efficacy between these agents and tamoxifen is comparable. Uncertainty remains concerning the select duration of therapy, the long-term toxicity profile, and the appropriate sequence of administration for AIs.4 Novel studies are needed to produce selective aromatase modulators that will produce less toxicity than the AIs.25,36-37
REFERENCES
1. Robertson JFR. Fulvestrant (FaslodexÆ)--how to make a good drug better. Oncologist . 2007;12:774-784.
2. Osborne CK. Drug therapy: tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618.
3. National Cancer Comprehensive Network. NCCN clinical practice guidelines in oncology--v.1.2008. Breast cancer. www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed October 11, 2007.
4. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol . 2005;23:619-629.
5. Dunn BK, Wickerham DL, Ford LG. Prevention of hormone-related cancers: breast cancer. J Clin Oncol. 2005;23:357-367.
6. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431-2442.
7. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol . 2001;19:881-894.
8. Lacy CF, Armstrong LL, Goldman MP, et al, eds. Drug Information Handbook. 13th ed. Hudson, OH: Lexi-Comp; 2005:117,598,871.
9. Ellis MJ, ed. Aromatase inhibitors for the treatment of breast cancer. Manhasset, NY: Oncology Publishing Group, CMP Healthcare Media; 2005:29-45.
10. Arimidex (anastrozole) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; May 2007.
11. Arora A, Potter JF. Aromatase inhibitors: current indications and future prospects for treatment of postmenopausal breast cancer. J Am Geriatr Soc. 2004;52:611-616.
12. Femara (letrozole) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; December 2006.
13. Shaw G. In preventing breast cancer occurrence...anastrozole beats tamoxifen in ATAC. Clin Oncol News . 1/2008. www.clinicaloncology.com/index.asp?ses=ogst§ion_id=148&show=dept&article_id=9884. Accessed March 17, 2008.
14. Aromasin (exemestane) package insert. New York, New York: Pfizer Inc; February 2007.
15. Baum M, Budzar AU, Cuzick J, et al, for the ATAC Trialists' Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131-2139.
16. Howell A, Cuzick J, Baum M, et al, for the ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60-62.
17. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793-1802.
18. Th¸rlimann B, Keshaviah A, Coates AS, et al, for the Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747-2757.
19. Coombes RC, Hall E, Gibson LJ, et al, for the Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:
1081-1092.
20. Ellis G, Bone HG, Chlebowski R, et al. A phase 3 study of the effect of denosumab therapy on bone mineral density in women receiving aromatase inhibitors for non metastatic breast cancer. Paper presented at: the 30th Annual San Antonio Breast Cancer Symposium; December 13-16, 2007; San Antonio, TX. Abstract 47.
21. Singh S, Cuzick J, Edwards R, et al. Effect of anastrozole on bone mineral density after one year of treatment: results from bone sub-study of the International Breast Cancer Intervention Study (IBIS-II). Paper presented at: the 30th Annual San Antonio Breast Cancer Symposium; December 13-16, 2007; San Antonio, TX. Abstract 28.
22. Dellapasqua S, Colleoni M, Gelber RD, Goldhirsch A. Adjuvant endocrine therapy for premenopausal women with early breast cancer. J Clin Oncol. 2005;23:1736-1750.
23. National Cancer Institute. Breast cancer treatment (PDQ). www.cancer.gov/cancertopics/pdq/treatment/breast/healthprofessional. Accessed December 26, 2007.
24. Baylor College of Medicine Clinical Trials. Suppression of Ovarian Function Trial (SOFT). www.breastcenter.tmc.edu/clinic/trials/soft.htm. Accessed December 26, 2007.
25. Aebi S, Castiglione-Gertsch M. Adjuvant endocrine therapy for the very young patients. Breast. 2003;12:509-515.
26. ClinicalTrials.gov. Suppression of ovarian function plus either tamoxifen or exemestane compared with tamoxifen alone in treating premenopausal women with hormone-responsive breast cancer. www.clinicaltrials.gov/. Accessed December 26, 2007.
27. Cuzick J. Aromatase inhibitors for breast cancer prevention. J Clin Oncol. 2005;23:1636-1643.
28. Karaer O, OruÁ S, Koyuncu FM. Aromatase inhibitors: possible future applications. Acta Obstet Gynecol Scand. 2004:83:699-706.
29. Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305-309.
30. Bulun SE, Yang S, Fang Z, et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79:19-25.
31. Feuillan P, Merke D, Leschek EW, Cutler GB. Use of aromatase inhibitors in precocious puberty. Endocr Relat Cancer. 1999;6:303-306.
32. Shozu M, Sebastian S, Takayama K, et al. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med. 2003;348:1855-1865.
33. Bowman A, Gabra H, Langdon SP, et al. CA 125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8:2233-2239.
34. Berstein L, Maximov S, Gershfeld E, et al. Neoadjuvant therapy of endometrial cancer with the aromatase inhibitor letrozole: endocrine and clinical effects. Eur J Obstet Gynecol Reprod Biol. 2002;105:161-165.
35. Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624-629.
36. Simpson ER, Dowsett M. Aromatase and its inhibitors: significance for breast cancer therapy. Recent Prog Horm Res. 2002;57:317-338.
37. Yue W, Santen RJ, Wang JP, et al. Aromatase within the breast. Endocr Relat Cancer. 1999;6:157-164.
To comment on this article, contact rdavidson@jobson.com.





